Topical Drug Delivery Market Size and Trends
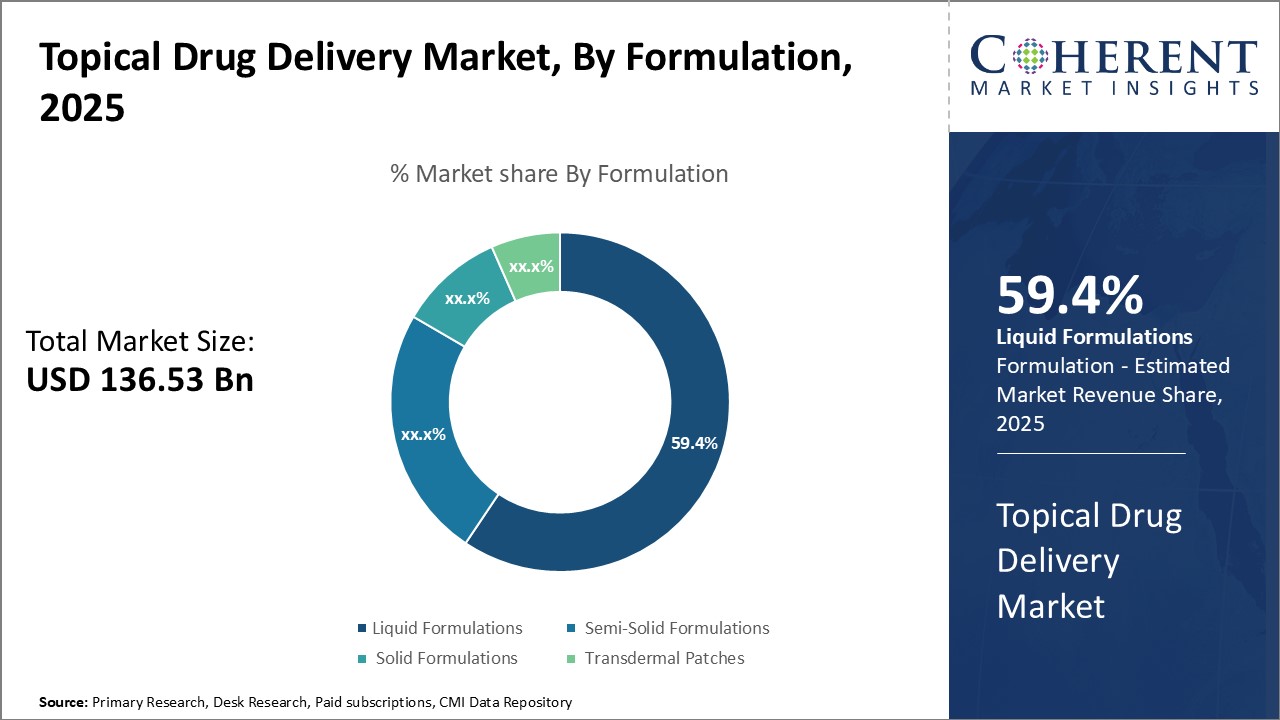
The topical drug delivery market is estimated to be valued at USD 136.53 Bn in 2025 and is expected to reach USD 254.59 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The increasing demand for pain relief drugs and over-the-counter products is driving the growth of the topical drug delivery market. The market is witnessing increased research and development activities focused on developing advanced topical drug delivery systems for dermatology and other therapeutic applications. Several top players are investing in developing novel topical formulations with enhanced permeation ability, sustained release, and targeted delivery to boost the efficiency of treatment. Nanotechnology and other advanced materials are being explored to improve topical drug delivery.
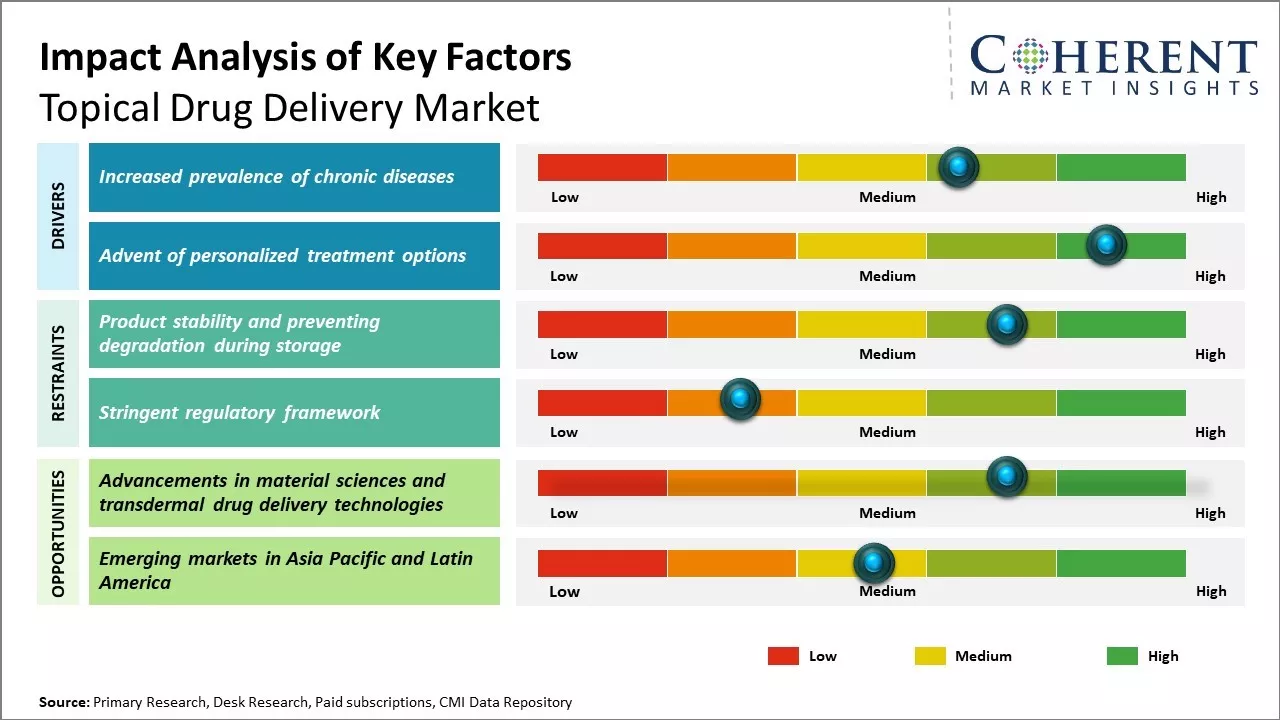
Increased prevalence of chronic diseases
The rising prevalence of chronic diseases across the world has been propelling growth within the topical drug delivery market. Chronic conditions, such as arthritis, dermatitis, acne, and other skin diseases, are becoming more widespread. This is owing to changing lifestyles and diet patterns along with increasing environmental pollution levels in both developed and developing regions. Topical drug delivery provides targeted treatment to the affected site of the skin, reducing systemic side effects. This is especially important for conditions where maintaining quality of life is critical. Creams, gels, and other topical formulations allow continuous localized therapy over long periods of time without major invasiveness. This is suitable for management of chronic pain and inflammation associated with arthritis and musculoskeletal disorders. Similarly, ointments and lotions effectively deliver medications to epidermis and provide relief from chronic skin diseases.
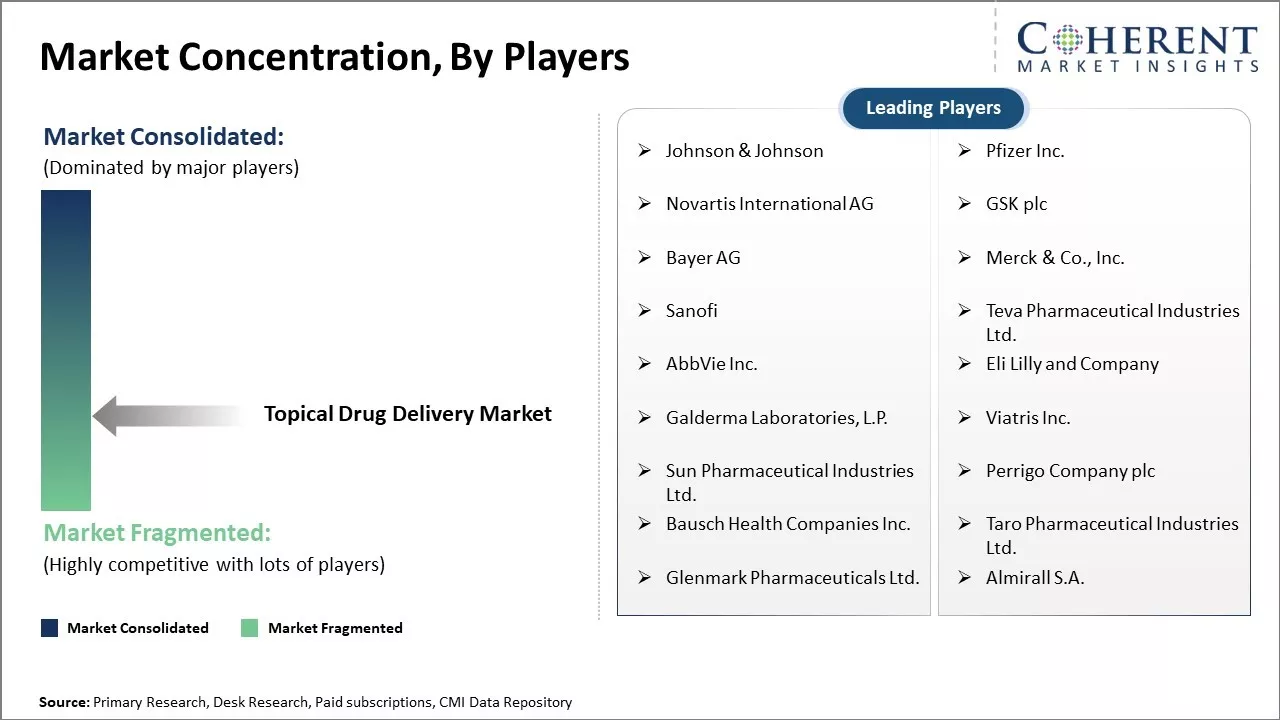
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Advent of personalized treatment optionsAnother important growth lever for the topical drug delivery market has been the advent of customized and personalized treatment solutions. Traditional topical therapies often involved a 'one-size-fits-all' approach. However, an individual's condition, genetics, and previous response to medications can vary significantly. There is a growing need to tailor treatment regimens based on a patient's unique biochemical characteristics, lifestyle habits, and disease risk factors. Advanced formulations leveraging nanotechnology, 3D printing, and transdermal drug delivery systems are enabling precise dosage and absorption levels. Wearable patches and implants are being designed to ensure steady, controlled release of active pharmaceutical ingredients for accurate dosing. Likewise, specialized gels, creams, and emulsions are being developed to penetrate specific skin layers and target distinct cell receptors based on molecular level profiling of the diseased area. These intelligent formulations achieve better efficacy rates while reducing the likelihood of systemic adverse effects and increasing patient compliance.

To learn more about this report, Download Free Sample
Market Challenges: Product stability and preventing degradation during storageThe topical drug delivery market faces several challenges. First, ensuring product stability and preventing degradation during storage remains an issue. Formulating drugs that can effectively penetrate the skin to reach the target site is another challenge, as the outer layers of skin act as a good barrier. Maintaining an even distribution of drugs on the application area and preventing accidental removal by external factors such as water or abrasion also challenge manufacturers.
Market Opportunities: Advancements in material sciences and transdermal drug delivery technologies
The non-invasive nature and self-application features of topical drugs increase patient compliance. Many newer drug molecules are now available that can selectively target deeper skin layers and tissues. Advancements in material sciences and transdermal drug delivery technologies now allow formulating more effective topical dosage forms. There is also scope to develop combination products that deliver multiple API simultaneously to treat complex conditions.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Formulation: Ease of application drives semi-solid formulations growthThe formulation segment includes liquid formulations, semi-solid formulations, solid formulations, and transdermal patches. Semi-solid formulations segment is anticipated to hold 59.4% of the market share in 2025. Semi-solid formulations such as ointments, gels, creams, and pastes offer optimal drug delivery for dermal administration. Their semi-solid yet spreadable consistency allows for easy application over the skin without being too runny or too thick. This ensures maximum contact of the active ingredients with the target site for effective treatment. Semi-solid formulations are also more emollient in nature compared to liquid formulations, providing moisturization to the skin. This added skin conditioning property enhances patient compliance. Further, semi-solid formulations form a protective layer on the skin that shields the active drug from external surroundings, thereby maintaining its stability and efficacy. Their semi-permeable nature also facilitates the controlled release of drugs into the skin for prolonged therapeutic effect. Given these advantages, healthcare professionals preferentially prescribe semi-solid drug formulations for dermatology conditions like acne, psoriasis, eczema, etc. driving their increased market uptake.
Insights, By Route of Administration: Convenience drives dermal segment growth
The route of administration segment includes dermal, rectal, vaginal, and others. Dermal contributed the highest share of the topical drug delivery market and is projected to hold 57.3% of the market share in 2025. The skin, being the largest organ of the body, provides a viable route for localized as well as systemic treatment of various diseases. Drugs administered dermally are delivered transdermally into the systemic circulation circumventing the gastrointestinal tract and liver, bringing down gastrointestinal side effects. This enhances patient comfort and compliance. Moreover, the dermal route localizes drug action at the site of application leading to fewer systemic side effects compared to oral medications. This makes it an attractive option for self-administration of chronic therapies. With growing awareness, healthcare professionals are increasingly preferring topical drug formulations targeting conditions affecting the epidermis and dermis like acne vulgaris, psoriasis, atopic dermatitis, fungal infections, etc. fueling the adoption of the dermal administration route.
Insights, By Distribution Channel: Convenience and product consultation drives retail pharmacies growth
The distribution channel segment includes hospital pharmacies, retail pharmacies, and online pharmacies. Retail pharmacies contributed the highest share of the topical drug delivery market and is projected to hold 49.3% of the market share in 2025. Being the first point of contact for healthcare, retail pharmacies are frequented more by patients compared to other channels for purchasing over-the-counter as well as prescription topical drugs. Their presence across geographical locations improves access for people in tier 2/3 cities and rural areas as well, expanding patient reach. Further, retail pharmacies employ trained pharmacists who are well-versed in advising patients on utilization and duration of topical treatments. This fosters greater customer loyalty and trust in their dermatology expertise. Retail pharmacies also offer product convenience via their shelf availability of a wide range of topical drugs from various brands. This product portfolio and one-stop-shop experience encourages patients to fulfill their topical medication needs at retail outlets driving their market dominance over other channels.
Regional Insights
Need a Different Region or Segment? Download Free Sample
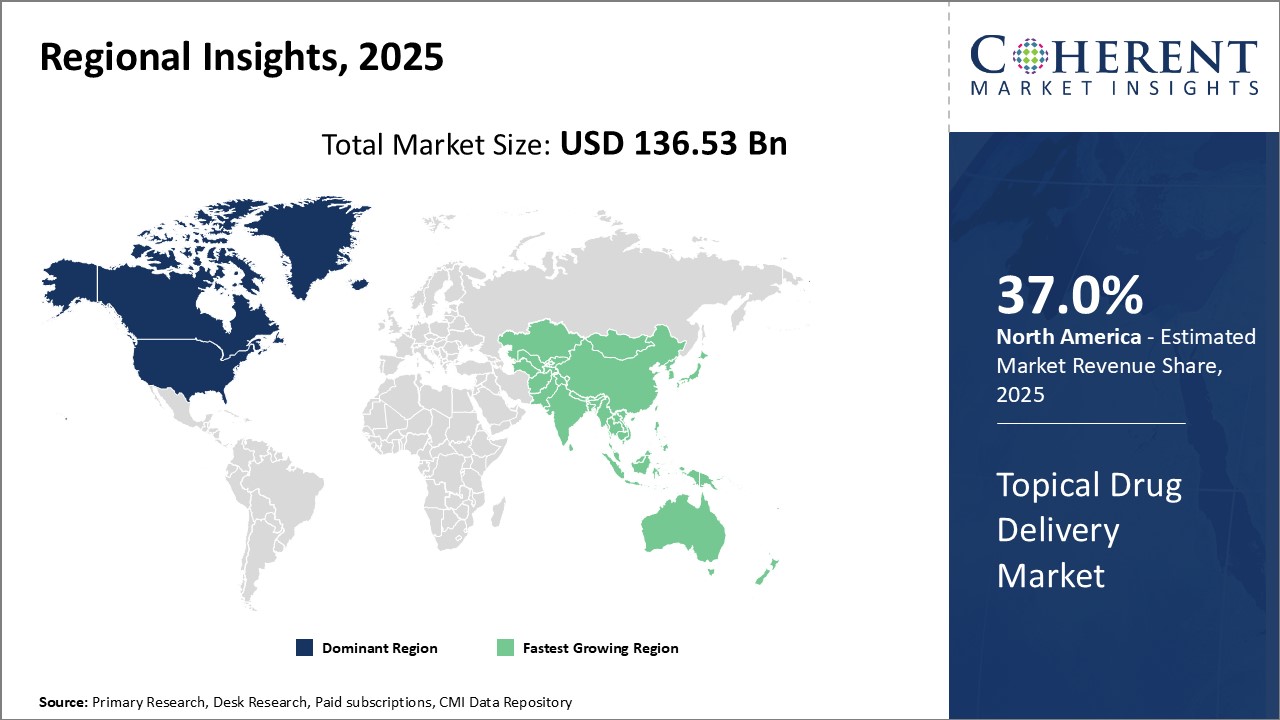
North America has dominated the topical drug delivery market over the past decade and is anticipated to hold 37.0% of the market share in 2025. With the strong presence of key pharmaceutical companies and many contract manufacturing organizations, the U.S. and Canada provide a robust research and development environment for topical drug formulations. The region is also a hotbed of innovation, with numerous startups working on advanced technologies, such as micro-needles, gels, and patches, to enhance skin permeation and the delivery of drugs. Stringent regulatory standards ensure the safety and efficacy of products, boosting overall market confidence. Manufacturers benefit from access to high quality raw materials and excipients from reliable local sources. The reimbursement environment also facilitates the adoption of premium topical drugs. Over-the-counter sales remain strong due to health-conscious consumer attitudes. These factors have enabled leading pharmaceutical companies to charge a premium for their patented topical products in North America.
Asia Pacific is emerging as the fastest growing region for topical drug delivery. Rapid economic development and rising incomes have increased healthcare spending across the region. Several Asian countries such as China, India, and Indonesia now have populations exceeding 100 million, representing massive patient pools for topical formulations. With growing expertise in generics manufacturing, Asian companies now supply affordable topical drugs globally. South Korea and Japan already have well-established pharmaceutical industries, and their populations have high demands for innovative skincare products. Meanwhile, India hosts facilities of most multinational drug firms and is a major exporter of topical generics. China's size and regulatory reforms present vast opportunities, though a lack of intellectual property protection remains a concern. However, as medical standards rise and income levels grow steadily every year, the Asia Pacific region is poised to play a much larger role in the global topical drug delivery landscape going forward.
Market Report Scope
Topical Drug Delivery Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 136.53 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 254.59 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Johnson & Johnson, Pfizer Inc., Novartis International AG, GSK plc, Bayer AG, Merck & Co., Inc., Sanofi, Teva Pharmaceutical Industries Ltd., AbbVie Inc., Eli Lilly and Company, Galderma Laboratories, L.P., Viatris Inc., Sun Pharmaceutical Industries Ltd., Perrigo Company plc, Bausch Health Companies Inc., Taro Pharmaceutical Industries Ltd., Glenmark Pharmaceuticals Ltd., and Almirall S.A. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Topical Drug Delivery Industry News
- On January 22, 2024, Arcutis Biotherapeutics, Inc., a commercial-stage biopharmaceutical firm, announced the launch of ZORYVE (roflumilast) topical foam, 0.3%, in the U.S. for the treatment of seborrheic dermatitis in people aged nine and ZORYVE is a once-daily steroid-free foam that is the first medicine approved for seborrheic dermatitis with a new mechanism of action in over two decades.
- In November 2023, NFlection Therapeutics Inc., a company developing topical MEK inhibitors for RAS-mediated skin conditions, announced positive topline results from a randomized, double-blind, vehicle-controlled Phase 2b clinical trial (NCT05005845). The trial evaluated NFX-179 Gel as a treatment for cutaneous neurofibromas (cNFs) in people with neurofibromatosis type 1 (NF1), a rare genetic condition.
- In June 2023, Novaliq GmbH, a biopharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has approved VEVYE (cyclosporine ophthalmic solution) 0.1% for the treatment of dry eye symptoms. VEVYE (development name CyclASol) is the first and only cyclosporine solution approved to treat the signs and symptoms of dry eye disease, with efficacy demonstrated within 4 weeks of treatment.
- In April 2022, Nobelpharma America, LLC, a pharmaceutical and medical device company, stated that the U.S. FDA has approved HYFTOR (sirolimus topical gel) 0.2% as the first topical treatment indicated for facial angiofibroma associated with TSC in adults and children aged six and above. HYFTOR is classified as an orphan drug for this indication.
*Definition: The topical drug delivery market provides products that deliver pharmacological agents through the skin or mucosa for both local and systemic effects. These products include creams, ointments, gels, lotions, and topical solutions or suspensions applied directly to the skin or mucosa. They are designed to allow active pharmaceutical ingredients to penetrate the delivery site at controlled rates and targeted depths in order to locally treat conditions or provide therapy to internal organs through transdermal delivery.
Market Segmentation
- Formulation Insights (Revenue, USD BN, 2020 - 2032)
- Liquid Formulations
- Semi-Solid Formulations
- Solid Formulations
- Transdermal Patches
- Route of Administration Insights (Revenue, USD BN, 2020 - 2032)
- Dermal
- Rectal
- Vaginal
- Others
- Distribution Channel Insights (Revenue, USD BN, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD BN, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Johnson & Johnson
- Pfizer Inc.
- Novartis International AG
- GSK plc
- Bayer AG
- Merck & Co., Inc.
- Sanofi
- Teva Pharmaceutical Industries Ltd.
- AbbVie Inc.
- Eli Lilly and Company
- Galderma Laboratories, L.P.
- Viatris Inc.
- Sun Pharmaceutical Industries Ltd.
- Perrigo Company plc
- Bausch Health Companies Inc.
- Taro Pharmaceutical Industries Ltd.
- Glenmark Pharmaceuticals Ltd.
- Almirall S.A.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
