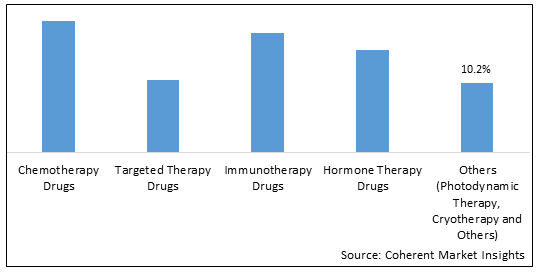
Skin Cancer Drugs Market is estimated to be valued at USD 10.3 Bn in 2025 and is expected to reach USD 18.00 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 8.3% from 2025 to 2032. Skin cancer drugs are used to treat melanoma and non-melanoma skin cancers. They can be administered through oral, topical, injectable, and other routes. The market is driven by rising incidence of skin cancers globally, growing awareness about early diagnosis and treatment, and launch of new targeted therapies. Global skin cancer drugs Market is segmented into drug type, cancer type, route of administration, distribution channel, and region. By drug type, the chemotherapy drugs segment accounts for the largest market share, owing to the high number of melanoma cases worldwide. Melanoma is the most dangerous form of skin cancer which accounts for a majority of skin cancer deaths.
Global Skin Cancer Drugs Market Regional Insights:
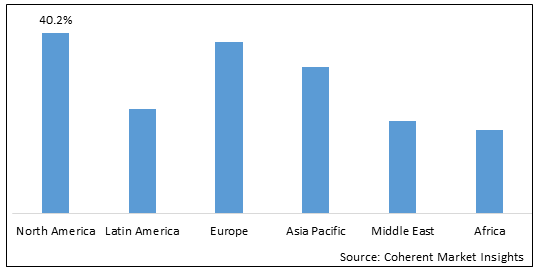
- North America is expected to be the largest market for skin cancer drugs market during the forecast period, which accounted for over 40.2% of the market share in 2025. The market growth in North America is due to the high incidence of skin cancers, advanced healthcare infrastructure, high awareness levels, and favorable reimbursement policies.
- The Europe market is expected to be the second-largest market for skin cancer drugs market, which accounted for over 30.1% of the market share in 2025. The market growth in Europe is due to the rising prevalence of skin cancers, presence of major players, and investments in research and development (R&D) activities.
- The Asia Pacific market is expected to be the fastest-growing market for skin cancer drugs market, with a CAGR of over 9.7% during the forecast period. The market growth in Asia Pacific is due to the improving healthcare infrastructure, growing disposable incomes, and increasing awareness about early diagnosis.
Figure 1. Global Skin Cancer Drugs Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst Views of the Global Skin Cancer Drugs Market:
- Global skin cancer drugs market is expected to grow significantly in the near future. The increasing prevalence of skin cancer worldwide which is driven by rising UV radiation exposure is a major driver fueling the demand for skin cancer treatment drugs. The growing awareness about skin cancer and various treatment options available is also supporting the growth of this market.
- However, the market still faces restraints from factors such as the high cost of targeted therapy drugs, lack of reimbursement policies, and low awareness in developing regions. The complexity of drug development and stringent regulations lengthens the time for new drug approvals, thereby hindering the market growth.
- The market provides opportunities for drugs with new mechanisms of action, combination therapies, and biosimilars. Asia Pacific is expected to be the fastest-growing region due to population growth, increased healthcare funding, and economic expansion. Within Asia, China and India are anticipated to offer high potential due to the large patient pool who is suffering from non-melanoma skin cancers.
- The melanoma segment dominates the global market. Nevertheless, the non-melanoma segment is likely to witness faster growth during the forecast period due to the rapidly increasing incidence rates of basal cell carcinoma and squamous cell carcinoma worldwide. Key market players could focus on investment and promotion in high-potential emerging markets to gain additional shares.
Global Skin Cancer Drugs Market Drivers:
- Increasing incidence of skin cancers: The rising incidence of skin cancers around the world is a major factor driving the growth of the skin cancer drugs market. Skin cancer is the most commonly diagnosed cancer globally. According to the estimates, in 2021 around 5.4 Mn new cases of non-melanoma skin cancers are diagnosed annually. Melanoma accounts for only a small portion of skin cancer cases but leads to a majority of skin cancer deaths. The global incidence of melanoma witnessed a significant rise over the last few decades. The increasing incidence is due to various factors like excessive Ultraviolet (UV) radiation exposure, genetic predisposition, growing geriatric population, and others. This growing patient pool represents a key driver fuelling the demand for skin cancer therapeutics. For instance, in 2022, according to the Cancer Research U.K., there are around 16,700 new melanoma skin cancer cases in the U.K. every year i.e. 46 patients every day are detected with melanoma.
- Launch of novel therapies: Pharmaceutical companies are increasingly focusing on development of novel targeted therapies and immunotherapies for skin cancer treatment. The approval and launch of innovative skin cancer drugs over the last few years has transformed the treatment landscape. Drugs like immune checkpoint inhibitors have shown positive results in clinical studies for advanced melanoma. Several targeted therapies for BRAF+ and NRAS+ mutant melanoma have also gained regulatory approvals. The launch of these advanced therapeutic agents has improved survival rates in skin cancer patients which are driving the market growth. In 2022, Immunocore Holdings plc., a commercial-stage biotechnology company pioneering the development of a novel class of T cell receptor (TCR) bispecific immunotherapies, announced that they received approval from the U.S. Food and Drug Administration (U.S. FDA) for KIMMTRAK (tebentafusp-tebn) which is being used for the treatment of HLA-A*02:01-positive adult patients with unresectable or metastatic uveal melanoma (mUM).
- Rising awareness and early diagnosis: Various public and private organizations are undertaking awareness campaigns about risk factors and symptoms of skin cancers globally. This is resulting in rising awareness among people about early diagnosis and timely treatment of the disease. People are increasingly adopting preventive measures like use of sunscreens. The higher awareness is leading to higher screening rates, early diagnosis and treatment initiation in skin cancer patients, -thereby driving the market growth. Improved understanding about personalized medicine is also contributing to the market growth.
- Favorable reimbursement: The availability of favorable reimbursement for skin cancer targeted therapies and immunotherapies in developed countries like the U.S. is facilitating uptake among patients. Medicaid and medicare provide coverage for majority of U.S. Food and Drug Administration (U.S. FDA) approved skin cancer drugs which reduces the out-of-pocket expenditure for patients. Private payers are also moving towards providing coverage for innovative therapies. The reimbursement support improves accessibility, affordability and compliance to newer skin cancer drugs, thus boosting the market growth.
Global Skin Cancer Drugs Market Opportunities:
- Emerging markets: Developing countries across Asia Pacific, Latin America, and Middle East &Africa represent significant untapped opportunities for skin cancer drugs market. This is due to the improving healthcare infrastructure, rising healthcare spending, and increasing awareness in these regions. The rising disposable incomes, healthcare reforms, and entry of global players in emerging economies is expected to increase adoption of branded therapies, thus -creating lucrative growth opportunities for market players in global skin cancer drugs market.
- Combination therapies: The potential of combination immunotherapies and targeted drugs is being increasingly evaluated in clinical trials for treating skin cancers. Combination of BRAF and MEK inhibitors has shown improved efficacy in patients as compared to single agent therapies. Checkpoint inhibitor combinations are also being assessed. The promising clinical data from combination therapy trials has highlighted the vast opportunity in this segment. Pharma companies are entering partnerships to boost their combinational clinical programs, which are expected to drive significant market growth.
- Advances in drug delivery: Advances in drug delivery approaches present significant opportunities in skin cancer drugs market. Topical dosage forms like creams, ointments, gels, patches allow direct delivery of drugs to the skin and thus improve compliance. Novel drug carriers like liposomes, nanoparticles, and nanoemulsions are being explored for improving skin penetration. Device aided delivery like microneedles and iontophoresis also offers growth potential for topical skin cancer therapies. Companies are increasingly investing in R&D for advanced drug delivery to tap into these opportunities.
- Biologics and biosimilars: The rising development and approval of biologics like monoclonal antibodies for skin cancer has widened the clinical pipeline with innovative therapies. Biosimilars of top selling biologics are also expected to enter the market post patent expiry, thus leading to expansion of affordable treatment options. The growth of the biologics and biosimilars segment is set to provide new opportunities for generic players and contract manufacturers in the skin cancer drugs supply chain.
Skin Cancer Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 10.3 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.3% | 2032 Value Projection: | USD 18.00 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis, Merck, Roche, Amgen, Pfizer, Sun Pharma, Bristol-Myers Squibb, AstraZeneca, Johnson & Johnson, Valeant, Daiichi Sankyo, Takeda, LEO Pharma, Mylan, Sanofi, Regeneron, Eli Lilly, Bayer, Gilead Sciences, Astellas Pharma |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Skin Cancer Drugs Market Trends:
- Increasing strategic partnerships: The recent years witnessed an increase in strategic partnerships between pharmaceutical giants, emerging biotech firms, and academic institutions for skin cancer drug development. Companies are collaborating to strengthen their product pipelines and combine their expertise. The deals also allow the companies to expand their skin cancer therapy portfolios by mutual technology access or joint molecule development. Moreover, the rising prevalence has encouraged cross-industry collaborations between pharmaceutical, dermatology, cosmetics and technology firms, thereby creating new avenues. For instance, in 2022, Regeneron Pharmaceuticals, Inc., a biotechnology company, purchased Sanofi's, a healthcare company, stake in the Regeneron and Sanofi collaboration on Libtayo (cemiplimab). Under this agreement, providing Regeneron Pharmaceuticals, Inc., with exclusive worldwide development, commercialization, and manufacturing rights to the medicine. The transaction is subject to merger control clearance outside the U.S. and was expected to close in the third quarter of 2022. Once the transaction closed, Regeneron recorded 100% of global net sales and expenses for the Libtayo program.
- Growing R&D focus on gene therapies: Researchers are increasingly focused on leveraging gene therapies to tackle advanced melanoma cases. Studies are underway to assess the potential of therapies like adoptive T-cell transfer, cancer vaccines, CRISPR-Cas9, and others. Clinical human trials are ongoing to evaluate gene treatments for skin cancers. Moreover, research on combinational approaches using gene therapy together with radiation, chemotherapy, targeted drugs, and others are gaining traction. The growing R&D emphasis on gene therapy is an emerging trend with potential to transform treatment landscape. For instance, on October 16, 2023, an Italy-Switzerland based biotechnology company Philogen and Sun Pharmaceutical, an India based multinational pharmaceutical company, announced positive results from the Phase III “Pivotal” trial of Nidlegy on locally advanced melanoma. Nidlegy is Philogen’s prorpietary product to treat skin cancer and is being investigated for the treatment of locally advanced melanoma, and for the treatment of high-risk basal cell carcinoma and other non-melanoma skin cancers.
- Shift towards personalized medicine: Recent research associated the response of skin cancer drugs with specific biomarkers and genomic mutation profiles in patients. As a result, emphasis on development of companion diagnostics is rising which can identify right patient population for targeted therapies which are based on personalized gene expression profiles. For instance, BRAF mutation screening is commonly conducted for prescribing BRAF inhibitors. The shift towards such stratified approaches is an important trend that can improve skin cancer outcomes.
- Growing adoption of technology: Artificial intelligence (AI), machine learning and data analytics are being leveraged to improve almost every aspect of skin cancer treatment, from diagnosis to post-treatment monitoring. Apps and dermatoscope devices enabled with AI algorithms can quickly screen lesions by analyzing images. Big data analytics helps generate insights to develop targeted drugs based on tumor mutation profile. Technology adoption is thus rising as an instrumental trend across the skin cancer drugs value chain.
Global Skin Cancer Drugs Market Restraints:
- High costs of targeted therapies: Most advanced targeted therapies and immunotherapies which are used for skin cancer treatment are premium priced, which makes affordability a major challenge. For instance, the average cost of PD-1 inhibitor pembrolizumab is around US$ 150,000 per patient annually. The high costs limit their uptake and also impact reimbursement coverage, especially in developing countries. The affordability issues hamper the growth potential of novel skin cancer drugs to some extent.
- Low awareness in developing regions: Despite with increasing incidences globally, the awareness about skin cancers remains low in developing countries of Asia, Africa, and Latin America. Lack of education about risk factors, inability to identify symptoms, limited access to dermatologists, and others are key challenges. Low awareness leads to delays in diagnosis and treatment initiation, thereby negatively impacting prognosis and survival outcomes. The poor awareness thus acts as a significant barrier to skin cancer drugs market growth in these geographies.
- Stringent regulatory guidelines: The various regional regulatory bodies like US FDA and European Medical Agency (EMA) have set very stringent safety and efficacy guidelines for approval of newer skin cancer therapies. The entire regulatory process of clinical trials and product approval requires huge financial investments and time. Delays in key pipeline drug approvals thereby restrain market expansion to some extent. Post-approval, the standards imposed around manufacturing, advertising, pharmacovigilance, and others also pose challenges for players.
- Counterbalance: The market players should focus on proper reimbursement policies for the patients, so that the high cost associated with the treatment of skin cancer can be performed without any reason for unaffordability.
Recent Developments:
New product launches:
- On March 22, 2023, Incyte, a U.S. based multinational pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) approved Zynyz (retifanlimab-dlwr), a humanized monoclonal antibody targeting programmed death receptor-1 (PD-1), for the treatment of adults with metastatic or recurrent locally advanced Merkel cell carcinoma (MCC)
- In 2021, Regeneron Pharmaceuticals, Inc., a U.S. basedbiotechnology company and Sanofi, a multinational pharmaceutical and healthcare company, announced that the U.S. Food and Drug Administration (FDA) approved the PD-1 inhibitor Libtayo (cemiplimab-rwlc) as the first immunotherapy treatment indicated for patients with advanced basal cell carcinoma (BCC), previously treated with a hedgehog pathway inhibitor (HHI) or for whom an HHI is not appropriate. Full approval was granted for patients with locally advanced BCC and accelerated approval was granted for patients with metastatic BCC.
- In June 2020, Regeneron Pharmaceuticals, a biotechnology company and Sanofi, multinational pharmaceutical and healthcare company gained U.S. FDA approval for Libtayo (cemiplimab-rwlc), a PD-1 inhibitor for the first-line treatment of patients with advanced non-small cell lung cancer (NSCLC). It significantly improved overall survival in advanced NSCLC patients.
Acquisition and partnerships:
- In 2022, Merck, a science and technology company, acquired cancer drug developer Imago BioSciences for US$1.35 billion. The acquisition strengthens Merck’s hematology portfolio with Imago BioSciences’ investigational candidate IMG-7289, an orally available lysine-specific demethylase 1 (LSD1) inhibitor being evaluated for myeloproliferative neoplasms.
- In 2017, Bristol Myers Squibb, a specialty biopharmaceutical company, entered into a clinical trial collaboration with AbbVie to evaluate the combination of Bristol Myers Squibb's PD-1 inhibitor Opdivo (nivolumab) with AbbVie's Navitoclax in patients with advanced solid tumors. The collaboration will boost their oncology clinical development programs.
Figure 2. Global Skin Cancer Drugs Market Share (%), By Drug Type, 2025
To learn more about this report, Download Free Sample
Top Companies in Global Skin Cancer Drugs Market:
- Novartis
- Merck
- Roche
- Amgen
- Pfizer
- Sun Pharma
- Bristol-Myers Squibb
- AstraZeneca
- Johnson & Johnson
- Valeant
- Daiichi Sankyo
- Takeda Pharmaceutical
- LEO Pharma
- Mylan N.V.
- Sanofi
- Regeneron
- Eli Lilly
- Bayer
- Gilead Sciences
- Astellas Pharma
*Definition: The skin cancer drugs market comprises of pharmaceutical products which are used for the treatment of various skin cancer indications including melanoma and non-melanoma skin cancers. It consists of chemotherapy, targeted therapy, immunotherapy, and other drugs used for skin cancer treatment.
Few Other Promising Reports in Pharmaceutical Industry:
Metastatic Melanoma Therapeutics Market
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




