Red Biotechnology Market is estimated to be valued at USD 910.26 Bn in 2025 and is expected to reach USD 1,854.38 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 10.7% from 2025 to 2032.
Red biotechnology involves the use of biological agents or organisms in the manufacturing of biopharmaceutical drugs, genetically modified crops, renewable biofuels, and other products. The key drivers of the red biotechnology market include rising prevalence of chronic diseases, increasing investments in R&D for biologics and biosimilars, and growth of the biopharmaceutical industry.
Global Red biotechnology market is segmented into by product type, application, end user, and region. By product type, the biopharmaceuticals segment accounted for the largest share of the market in 2025. Biopharmaceuticals include protein-based therapeutics such as monoclonal antibodies, insulin, and growth hormones that are manufactured by using living organisms. Rising incidence of chronic illnesses such as cancer, autoimmune disorders, and diabetes boosts demand for biopharmaceuticals.
Global Red Biotechnology Market- Regional Insights
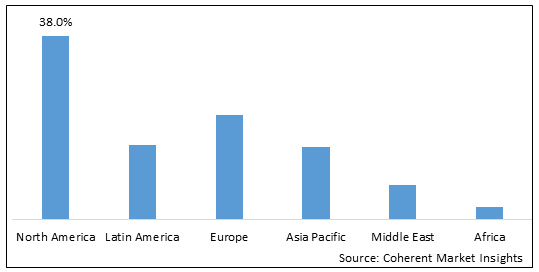
- North America is the largest market for red biotechnology, accounting for over 38% of the global market in 2025. The growth of the red biotechnology market in North America is driven by a number of factors including:
- The presence of a large number of red biotechnology companies
- High investment in red biotechnology research and development
- Government support for the red biotechnology industry
- Europe is the second largest market for red biotechnology, accounting for over 30% of the global market in 2025. The growth of the red biotechnology market in Europe is being driven by a number of factors, including:
- Increasing prevalence of chronic diseases such as cancer, cardiovascular disease, and diabetes
- Growing demand for personalized medicine
- Government support for the red biotechnology industry
- Asia Pacific is the fastest growing market for red biotechnology, accounting for over 20% of the global market in 2025. The growth of the red biotechnology market in Asia Pacific is being driven by a number of factors including:
- Increasing prevalence of chronic diseases such as cancer, cardiovascular disease, and diabetes
- Growing demand for personalized medicine
- Rising investments in red biotechnology research and development
- Government support for the red biotechnology industry
Figure 1. Global Red Biotechnology Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst View
- The global red biotechnology market is expected to experience significant growth over the forecast period driven by the increasing investment in R&D of monoclonal antibodies and recombinant proteins. North America currently dominates the market owing to supportive government initiatives and presence of key market players in the region. However, Asia Pacific is likely to emerge as the fastest growing market with countries like China and India expanding their biologics manufacturing capabilities. The market is witnessing a shift towards development of biosimilars aiming to reduce healthcare costs which is expected to create new opportunities for players. Nonetheless, intellectual property issues related to patent cliffs of major biologics pose a threat to innovator companies. Any delay in drug approvals or clinical trials can negatively impact revenues of players. High costs associated with R&D and clinical trials of biologics remains a major challenge for small and mid-sized companies. Regulatory hurdles for approval and commercialization of biosimilars varies across countries acts as a deterrent for global players. Overall, the market is expected to benefit from the growing demand for effective treatments for chronic diseases and cancer. Moreover, collaborations between pharmaceutical companies and research institutions are accelerating the drug development process. Adoption of personalized medicine and combination therapies will further boost market growth. However, players need to focus on demonstrating cost
Global Red Biotechnology Market- Drivers
- Increasing Prevalence of Chronic Diseases: Rising prevalence of chronic illnesses such as cancer, cardiovascular diseases, diabetes, and autoimmune disorders boosts demand for novel biologics. According to article published by WHO on September 16, 2023, chronic diseases account for 74% of deaths globally. Biologics can offer targeted action for such diseases as compared to traditional small molecule drugs. Companies are developing antibodies, cell therapies, and other biologics for immuno-oncology, autoimmune disorders like rheumatoid arthritis, and diabetes. With the increasing disease burden, sales of approved biologics and the pipeline of drugs under development is continuously expanding, and this is propelling the growth of the red biotechnology market
- Growth in Precision Medicine: The emerging field of precision medicine and rising emphasis on targeted therapeutics tailored to an individual’s genetics is fueling R&D in biologics. Because of their ability to interact with complicated signaling proteins and regulate disease pathways based on a patient's genetic profile, biologics lend themselves well to precision medicine. Biopharma businesses are utilizing biotechnology to generate precision medicines ranging from CAR T-cell treatments for blood malignancies to tailored cancer vaccines. Government measures in countries such as the United States and the European Union to boost precision medicine research auger well for biotech businesses seeking to hasten the development of such focused medications.
- Expanding Biopharmaceutical Industry: The rapidly expanding biopharmaceutical industry, increasing number of new drug approvals, and rising manufacturing of biologics are key factors governing growth of red biotechnology. According to IQVIA, provider of advanced analytics, technology solution, biologics accounted for 26% of global pharma sales in 2021. The sales revenue from biotech drugs is projected to grow steadily due to higher drug development productivity and expanding treatment population. Furthermore, increasing approvals of biosimilars across the globe is widening the clinical reach of biologics. Overall, the proliferating biopharma sector underpinned by biologics adoption is a primary driver for the red biotechnology market.
Red Biotechnology Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 910.26 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.7% | 2032 Value Projection: | USD 1,854.38 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., Gilead Sciences, Inc., Biogen, Pfizer Inc., Novartis AG, F. Hoffmann-La Roche, Johnson & Johnson Services, Inc., Sanofi, Merck & Co. Inc., AbbVie Inc., GSK plc., AstraZeneca, Eli Lilly and Company, Novo Nordisk A/S, Bayer AG, Bristol-Myers Squibb Company, Teva Pharmaceutical Industries Ltd., Takeda Pharmaceutical Company Limited, Boehringer Ingelheim International GmbH and Astellas Pharma Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Red Biotechnology Market- Opportunities
- Emerging Markets: The growing pharmaceutical markets in emerging countries across Asia Pacific, Latin America, and Middle East & Africa present significant opportunities for red biotechnology. Improving healthcare infrastructure, rising health expenditure, and increasing insurance coverage are making innovative biologics more accessible in these regions. Companies are expanding their emerging market presence through licensing deals with local partners, and focusing on biotherapeutics to treat population-specific For instance, in the year 2019, AstraZeneca has expanded its biomedicines portfolio in China. Such efforts can increase the availability of novel biologics in high potential emerging markets, and will open new avenues for growth.
- Adoption of Advanced Manufacturing: The growing adoption of advanced manufacturing technologies such as single-use bioreactors, continuous processing, and modular facilities provide new opportunities for biomanufacturing scale-up and efficiency. Single-use systems enable flexibility, lower capital costs, and risk mitigation. Automation solutions allow real-time process monitoring and control. Companies investing to upgrade production with such technologies can maximize output, reduce costs, and ensure smooth bioprocessing, thereby gaining competitive advantage.
- Biologics for New Therapeutic Areas: The application scope of biologics is expanding beyond the traditional areas of cancer and immunology into new domains like neurodegenerative disorders. Biogen’s Aduhelm recently became the first approved Alzheimer’s drug targeting amyloid beta. The drug was approved by U.S. Food and Drug Administration in the year 2021. The drug demonstrated biomarkers clearing amyloid plaques. These are increasing biologics R&D for untapped areas with high unmet demand. Furthermore, new delivery systems like as intrathecal injection improve targeting. As companies use biotech to discover medicines for unmet ailments, enormous opportunities for market development may emerge.
Global Red Biotechnology Market- Trends
- Growing Adoption of Personalized Medicine: The adoption of personalized medicine using biomarkers, genetics, and clinical data to customize treatments for patients is a rising trend benefiting red biotechnology. Companies are actively researching targeted biologics and precision immunotherapies that can selectively attack diseased cells based on a person's molecular profile. For example, Vitrakvi is a tissue-agnostic precision therapy for cancers with NTRK gene fusion. The future prospects of personalized medicine are immense, with the potential to make therapies more efficacious and safe.
- Strategic Collaborations on Novel Platform Technologies: In recent years, more cross-industry collaborations are being forged to combine complementary capabilities in bioprocessing, gene therapy, drug delivery, and manufacturing. Gilead-Galapagos, GSK-23andMe, Evotec-Bayer are some partnerships offering integrated expertise to fast-track innovation. Small biotech gain market access while big pharmas reduce risks and costs through synergy. Such mutually beneficial strategic tie-ups leverage novel platform technologies to accelerate biotherapeutics discovery and development.
- Rising Adoption of AI and Big Data: Pharma companies are increasing the use of big data analytics and AI to streamline biologics R&D. AI helps to identify new disease targets, predict clinical outcomes, design biologics, and simulate trials - compressing timelines and costs Data analytics improves biomanufacturing yields. Cloud-based informatics integrates workflows. The application of advanced computing can optimize biopharmaceutical processes and boost productivity.
- Shift to Low-Cost Biosimilar Drugs: Given the high costs of innovator biologics, there is a clear shift towards more affordable biosimilars which provide comparable efficacy and safety. The U.S. FDA approved 31 biosimilars between 2017 and 2021. Key biologic patents are also beginning to expire, opening the door for competition. Scaling biosimilar adoption can expand access to essential treatments while reducing pharmaceutical spending - a trend benefiting healthcare systems globally. Companies are strategizing to capitalize on the multi-billion dollar potential.
Global Red Biotechnology Market- Restraints
- Pricing Pressures on Biologics: Although biologics represent a fast-growing segment, these are facing increasing pricing and reimbursement pressures. Due to their high costs, payers are implementing measures like shifting a higher cost burden to patients to control expenditures. Competition from biosimilars is also introducing pricing challenges for branded biologics. Companies may have to reassess premium pricing strategies in the long run to balance revenues with affordability, especially in emerging economies.
- Long and Capital Intensive Development: The R&D process for biologics is prolonged, risky and requires substantial capital investments, and this acts as a market restraint. Complex large molecule drugs take 10-15 years to go from lab to market with high failure rates. The costs for bringing a new biologic to market can exceed US$ 2.5 billion. Extensive clinical trials and manufacturing challenges add to development timeframes. The huge time and financial commitments deter many smaller companies from biotherapeutic innovation.
- Stringent Regulatory Requirements: The biologics regulatory pathway involves exhaustive analytical characterization, immunogenicity testing and monitoring requirements, presenting a restraint for manufacturers. The FDA and EMA have high approval standards to ensure safety given the sensitivity of biological products. Manufacturing facilities and quality control procedures also undergo rigorous scrutiny. Added regulatory burdens of demonstrating biosimilarity further complicate the process. Meeting evolving compliance criteria requires significant investments.
Global Red Biotechnology Market- Recent Developments
New Product Launches
- On April 27, 2023, Pfizer Inc., a pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) had approved PREVNAR 20(20-valent Pneumococcal Conjugate Vaccine) for the prevention of invasive pneumococcal disease (IPD) caused by the 20 Streptococcus pneumoniae (pneumococcal) serotypes contained in the vaccine in infants and children six weeks through 17 years of age, and for the prevention of otitis media in infants six weeks through five years of age caused by the original seven serotypes contained in PREVNAR
- In September 2021, BDR Pharmaceuticals, a pharmaceutical company launched generic Cabozantinib that is used for the treatment of various types of cancer in India. Cabozantinib is used for the treatment of metastatic medullary thyroid cancer, advanced renal cell carcinoma, and hepatocellular carcinoma.
- In May 2020, U.S. Food and Drug Administration approved atezolizumab in combination with bevacizumab manufactured by Genentech Inc, a biotechnology corporation, for patients with unresectable or metastatic hepatocellular carcinoma who have not received prior systemic therapy
- In March 2020, U.S. Food and Drug Administration granted accelerated approval to the combination of nivolumab and ipilimumab manufactured by Bristol-Myers Squibb Co., a global biopharmaceutical company, for patients with hepatocellular carcinoma (HCC) who have been previously treated with sorafenib
Acquisition and Partnerships
- On January 9, 2023, Ipsen, a France-based biopharmaceutical company, and Albireo, a biotech company providing pharmaceutical products and services, announced that both had signed a binding merger agreement, under which Ipsen would acquire Albireo, a pioneer in the development of bile-acid modulators, for the treatment of cholestatic liver disorders in both children and adults
- In November 2022, TriSalus Life Sciences, a privately held oncology therapeutics company, announced that it had entered into a definitive merger agreement with MedTech Acquisition Corporation, a publicly traded special purpose acquisition company
- In August 2022, GSK plc, a pharmaceutical company, announced that it had completed the acquisition of Affinivax, Inc (Affinivax), a U.S.-based clinical-stage biopharmaceutical company. Affinivax has pioneered the development of a novel class of vaccines, the most advanced of which are next-generation pneumococcal vaccines.
- In June 2020, Gavi, the Vaccine Alliance’s, an independent multilateral funding organization, announced a new supply agreement with Serum Institute of India, Pvt. Ltd. (SIIPL), a pharmaceutical company, under the Advance Market Commitment (AMC) for its World Health Organization prequalified pneumococcal conjugate vaccine (PCV), PNEUMOSIL, to fight against pneumococcal disease, a leading cause of severe childhood pneumonia, sepsis, and meningitis in the low and middle income countries such as India, Bangladesh, and others
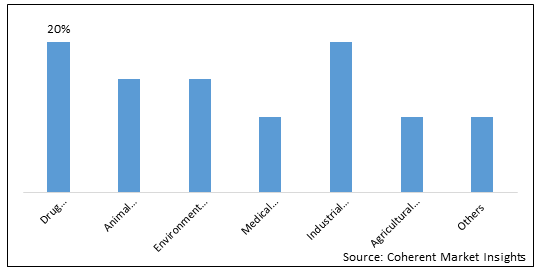
Figure 2. Global Red Biotechnology Market Share (%), By Application, 2025
To learn more about this report, Download Free Sample
Top companies in Global Red Biotechnology Market
- Amgen Inc.
- Gilead Sciences, Inc.
- Biogen
- Pfizer Inc.
- Novartis AG
- Hoffmann-La Roche
- Johnson & Johnson Services, Inc.
- Sanofi
- Merck & Co. Inc.
- AbbVie Inc.
- GSK plc.
- AstraZeneca
- Eli Lilly and Company
- Novo Nordisk A/S
- Bayer AG
- Bristol-Myers Squibb Company
- Teva Pharmaceutical Industries Ltd.
- Takeda Pharmaceutical Company Limited
- Boehringer Ingelheim International GmbH
- Astellas Pharma Inc.
*Definition: Red biotechnology involves the use of biological agents or organisms in the manufacturing of biopharmaceutical drugs, genetically modified crops, renewable biofuels, and other products. It includes disciplines like genetics, molecular biology, biochemistry, embryology and cell biology that are used to develop products and services in sectors like human and veterinary healthcare, agriculture, food production, and environmental management. Red biotechnology has applications in fields such as drug development, gene therapy, tissue engineering, diagnostics, and vaccine development. The most prominent application is in the development of biopharmaceuticals like monoclonal antibodies, hormones, and antigens used for treating diseases like cancer, autoimmune disorders, and infections. Red biotechnology is also used in agriculture to genetically modify crops, enhance yield, and improve disease resistance. Overall, red biotech aims to harness biological and living systems to create commercial products that can benefit mankind.
Few other promising reports in Biotechnology Industry
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




