Preterm Birth and PROM Testing Market is estimated to be valued at USD 1.92 Bn in 2025 and is expected to reach USD 2.94 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 6.3% from 2025 to 2032.
Preterm birth and Premature Rupture of Membranes (PROM) testing involves various medical tests to check for the risk of preterm delivery in pregnant women. It helps in early diagnosis and timely clinical intervention to reduce preterm births and associated complications. The market is driven by the rising prevalence of preterm births, advancements in prenatal testing, and government initiatives to improve prenatal care.
The preterm birth and PROM testing market is segmented based on product type, test type, end user, and region. By test type, the market is segmented into pelvic exam, ultrasound, uterine monitoring, biomarker testing, and others. The ultrasound segment accounted for the largest market share in 2025 owing to the wide usage of ultrasound scans for detecting risks associated with preterm birth.
Preterm Birth and PROM Testing Market Regional Insights
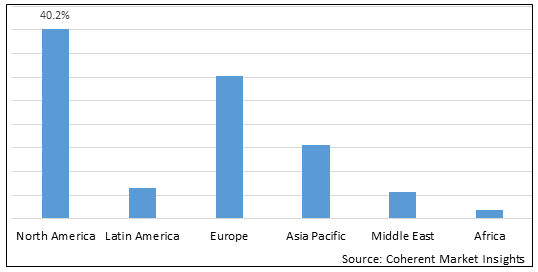
- North America is expected to be the largest market for preterm birth and PROM testing during the forecast period, accounting for over 40.2% of the market share in 2025. The growth of the market in North America is attributed to the high prevalence of preterm births, presence of major players, and increasing adoption of innovative prenatal screening tests in the region. For instance, according to Centers for Disease Control and Prevention, in the U.S., around one in 10 newborns were affected by preterm birth in 2022. Preterm birth rates in the U.S. in 2022 were almost 50% higher among African-American women (14.6%) than among White or Hispanic women (9.4% and 10.1%, respectively).
- Europe is expected to be the second-largest market for preterm birth and PROM testing, accounting for over 32.3% of the market share in 2025. The growth of the market in Europe is attributed to the rising awareness about prenatal testing, government funding for prenatal screening programs, and advancing healthcare infrastructure in the region.
- Asia Pacific market is expected to be the fastest-growing market for preterm birth and PROM testing, and it is projected to grow at a CAGR of over 15.1% during the forecast period. The growth of the market in Asia Pacific is attributed to the large target population, improving healthcare expenditure, and growing investments by leading companies in the region.
Figure 1. Global Preterm Birth and PROM Testing Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Analyst’s Views
The preterm birth and PROM Testing Market is expected to grow steadily driven by the increasing rate of preterm births worldwide. The rising health awareness and focus on prenatal care among expectant mothers are further driving the demand for PROM tests and diagnostic tools to identify the risks of preterm delivery. North America currently dominates the market owing to the well-established healthcare infrastructure and advanced diagnostic facilities in the region. However, Asia Pacific is likely to emerge as the fastest growing regional market in the coming years backed by the growing population, improving access to healthcare, and rising medical tourism in countries such as India and China.
Preterm Birth and PROM Testing Market Drivers
- Increasing prevalence of preterm births: The rising prevalence of preterm births is a major factor driving the growth of the preterm birth and PROM testing market. Globally, as per United Nations, around one in 10 babies are born prematurely every year. Complications from preterm births are the leading cause of death among children under age 5. The increasing rate of preterm deliveries has led to greater demand for reliable diagnostic tests to screen and monitor high-risk pregnancies. Usage of prenatal testing tools helps in the early detection of risks and timely clinical intervention to improve health outcomes.
- Technological advancements in prenatal screening and diagnosis: Continuous advancements in prenatal screening and diagnostic technologies, such as ultrasound scans, biomarker tests, and home-based pregnancy tests, are contributing to the growth of the preterm birth and PROM testing market. Next-generation gene sequencing, cell-free DNA analysis, biochemical assays, and microfluidics allow detection of preterm birth risks with greater sensitivity and accuracy. Key companies are focused on developing innovative products integrated with advanced technologies to improve prenatal testing capabilities. This is expected to increase the adoption of upgraded prenatal testing products and solutions. For instance, in June 2022, Mylab Discovery Solutions, a biotechnology company, launched PregaScreen, an at-home pregnancy test kit. The kit provides accurate results within a few minutes and can be purchased over-the-counter at local pharmacies across India.
- Favorable government policies and reimbursement framework: Expanding government investments to improve prenatal care facilities and services are fostering the adoption of preterm birth and PROM testing globally. Government-led prenatal screening programs, favorable reimbursement policies, growing awareness initiatives, and funding for R&D in prenatal diagnostics are encouraging the development and commercialization of advanced preterm birth risk assessment products. This creates lucrative growth opportunities for market players.
- Increasing demand for personalized medicine: The growing trend of personalized medicine is stimulating the preterm birth and PROM Testing Market growth. Prenatal screening tests allow risk stratification and enable physicians to design patient-centric management plans. Diagnosing and predicting preterm birth risks by assessment of genetic predisposition and physiological factors allows timely interventions customized to the specific health needs of expectant mothers. This is anticipated to propel the demand for personalized prenatal screening.
Preterm Birth and PROM Testing Market Opportunities
- Significant untapped potential in emerging economies: Developing nations across Asia Pacific, Latin America, and Africa represent untapped markets with huge growth potential for preterm birth and PROM testing products owing to the high birth rate and rising healthcare expenditure in these regions. Improving reimbursement scenarios, penetration of cutting-edge technologies, and growing health awareness are anticipated to PROMote the adoption of innovative prenatal diagnostic tools and services. Players can focus on collaborations, partnerships, and geographical expansion to tap into these high-growth markets.
- Integration of AI and big data analytics: Incorporation of emerging technologies like Artificial Intelligence (AI), machine learning, and big data analytics in prenatal testing products for intelligent risk assessment and predictive insights represents a significant opportunity for market growth. Platforms combining advanced algorithms, fetal medicine expertise, and patient data analysis will allow accurate quantification of preterm birth risks and support proactive clinical management. Key players can leverage these technologies to develop smarter, personalized prenatal screening solutions.
- Development of direct-to-consumer testing models: The expanding adoption of at-home pregnancy testing and the emergence of direct-to-consumer prenatal screening options is creating lucrative avenues for preterm birth and PROM testing products. Key players can focus on over-the-counter and home-based solutions designed for in-home sample collection and virtual result consultation. User-friendly self-testing kits that enable remote risk assessment and monitoring of high-risk pregnancies hold immense potential for growth.
- Strategic collaborations and acquisitions: The preterm birth and PROM testing space is witnessing rising collaborations, partnerships, and mergers, acquisitions among leading diagnostics companies, research institutions, and technology firms. Strategic deals focused on expanding product portfolios, entering new markets, and technology integration can significantly elevate companies’ market standing and commercialization capabilities. Collaborative models for large-scale clinical studies and product development also support growth. For instance, in August 2021, BioReference Laboratories, Inc., an OPKO Health company, announced acquisition of Roche's centralized laboratory prenatal testing business in the U.S. The Harmony Prenatal Test, developed by Ariosa, is one of the most extensively researched non-invasive prenatal screening (NIPS) tests used in prenatal screening.
Preterm Birth And PROM Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.92 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.3% | 2032 Value Projection: | USD 2.94 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Biosynex, Qiagen N.V., Clinical Innovations, LLC, Sera Prognostics, Hologic, Inc., IQ Products, NX Prenatal, Inc., Promega Corporation, Insight Pharmaceuticals LLC, and Creative Diagnostics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Preterm Birth and PROM Testing Market Trends
- Shift toward point-of-care and self-testing models: Point-of-care and home-based diagnostic testing platforms are gaining PROMinence in the preterm birth and PROM Testing Market owing to their simplicity, convenience, and rapid results. Handheld devices and self-testing kits enabling quick risk assessment and timely intervention at the convenience of home are witnessing high adoption. Market players are increasingly focusing on developing patient-centric solutions for decentralized testing. The POC and self-testing trend is expected to continue gaining momentum during the forecast period. For instance, in November 2020, Nixxi, a women's health care company, announced the launch of PopNatal, a noninvasive, self-administered digital tool for women to immediately discover their risk for premature delivery. PopNatal swiftly and simply screens data with a unique chatbot interface.
- Use of multiple biomarkers for risk prediction: Advancements in omics and systems biology approaches are enabling the discovery of novel biomarkers associated with preterm birth risks. The market is gradually moving away from single biomarker-based tests toward multiplex biomarker panels and molecular signatures for accurate prediction of preterm birth likelihood. Next-generation tools analyzing combinations of biomarkers, demographic information, and clinical data will likely become the future of prenatal risk screening. For instance, in November 2022, WHOOP, a human performance company, launched a first-of-its-kind pregnancy digital biomarker for noninvasive prescreening for premature birth.
- Influx of new products with superior accuracy: Key diagnostics companies are investing significantly in R&D initiatives to introduce innovative products integrated with cutting-edge technologies to enhance accuracy and reliability of preterm birth risk assessment. New product launches focused on early screening, predictive capabilities, and greater sensitivity, such as premature birth indicators, advanced NIPT assays, wearable uterine activity monitors, hold immense commercialization potential. The influx of novel prenatal testing solutions is expected to intensify during the coming years.
- Growing industry focus on expanding the prenatal test portfolio: Leading diagnostics manufacturers are focused on diversifying their offerings of reproductive and prenatal genetic screening tests including carrier screening, NIPT, and newborn testing. Strategic developments through collaborations, partnerships, and acquisitions concentrated on adding new testing capabilities to leverage cross-selling opportunities. The ability to provide a comprehensive panel of prenatal assessments for complete pregnancy care is becoming an important competitive edge. This trend is likely to shape the market dynamics during the forecast period.
Preterm Birth and PROM Testing Market Restraints
- High cost of prenatal diagnostic tests: Despite favorable reimbursement policies, the high cost of advanced prenatal screening solutions, such as NIPT and genetic testing, is a key factor limiting the adoption of these tests in low and middle-income countries. Additionally, out-of-pocket expenditure remains high in developing regions with inadequate insurance coverage and accessibility constraints. This impedes market growth to some extent. Players are increasingly developing cost-effective rapid diagnostic kits and expanding access programs to address affordability challenges. For instance, according to NCBI, the cost of Non-invasive Prenatal Testing ranges from US$ 800 to US$ 2,000 in the U.S. and from US$ 500 to US$ 1,500 elsewhere.
- Shortage of trained professionals in developing regions: The shortage of trained medical professionals specializing in prenatal care in rural areas and low-resource settings of emerging countries negatively impacts the adoption of innovative preterm birth testing tools and technologies. Lack of knowledge regarding best practices in prenatal screening and diagnosis also contributes to hesitation among physicians against using new products without proper training. Players are conducting awareness programs and workshops focused on prenatal testing to help overcome this barrier.
- Low sensitivity of traditional diagnostic methods: Traditional diagnostic approaches for preterm birth risk screening, such as ultrasound monitoring and pelvic exams, exhibit low sensitivity and accuracy in predicting preterm delivery. This limits their reliability for timely and proactive interventions during pregnancy. However, advancements in omics, biomarker discovery, and Artificial Intelligence (AI)-based risk quantification are helping enhance the capabilities of modern prenatal screening solutions. This trend will help address limitations of conventional diagnostic techniques in the coming years.
Recent Developments
New product launches
- In April 2023, Mediclinic Southern Africa, a private hospital company, announced the launch of safe, non-invasive prenatal testing (NIPT) for pregnant parents as part of its DNA-based diagnostic and clinical interpretation services. This is a new product that was added once Mediclinic Ancestry testing was introduced in 2023.
- In October 2022, Ambry Genetics, a clinical diagnostic testing company and a division of REALM IDx, a biotechnology company, launched a new reproductive health initiative powered by its Comprehensive Assessment Risk and Education (CARE) Program, a digital platform designed to increase access to non-invasive prenatal testing (NIPT), also known as carrier screening, and support patients in making decisions.
- In July 2022, GE Healthcare, a medical technology company, launched its most advanced ultrasound, the next-generation Voluson Expert 22. This latest addition to GE Healthcare’s award-winning Women’s Health portfolio utilizes graphic-based beam former technology, which produces higher quality images and offers greater flexibility in imaging functions during pregnancy.
Acquisitions and partnerships
- In February 2022, Sera Prognostics Inc., a pregnancy company focused on improving maternal and neonatal health by providing innovative pregnancy biomarker, announced the execution of a partnership with MultiPlan, a healthcare management company. The agreement will help expand access to the PreTRM Test, the company's proprietary proteomic blood test for measuring a woman's risk of spontaneous preterm birth.
- In January 2022, QIAGEN N.V., a clinical diagnostic company, announced a partnership with Atila BioSystems, a biotechnology company, to provide non-invasive prenatal testing (NIPT) solutions to QIAGEN N.V’s dPCR franchise. NIPT requires only a blood sample from the mother, and replaces more invasive testing methods such as amniocentesis that can endanger the fetus and mother.
- In June 2019, Invitae Corporation, a medical genetics company, announced that it has entered into a definitive agreement to acquire Singular Bio, Inc., a privately-held company developing single molecule detection technology that enables lower costs and expanded use of high-quality, cell-free, nucleic acid analysis, initially for application in non-invasive prenatal screening (NIPS)
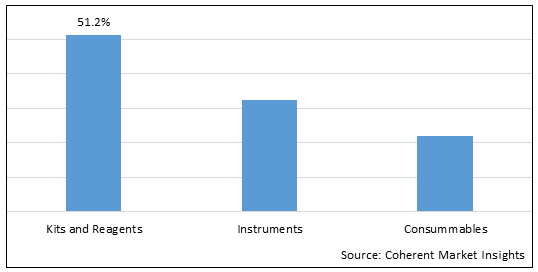
Figure 2. Global Preterm Birth and PROM Testing Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Top Companies in the Preterm Birth and PROM Testing Market
- Abbott Laboratories
- Biosynex
- Qiagen N.V.
- Clinical Innovations, LLC
- Sera Prognostics
- Hologic, Inc.
- IQ Products
- NX Prenatal, Inc.
- Promega Corporation
- Medical predictive Technologies Inc.
- Creative Diagnostics
Definition: The preterm birth and PROM testing market refers to the market and domain of diagnostic tests and tools used to detect the risk of preterm birth and premature rupture of membranes (PROM) in pregnant women. It includes various prenatal screening and diagnostic products such as pelvic exams, ultrasound devices, biomarker testing kits, pH test kits, fetal fibronectin tests, ferning test tools, and PROM detection kits. The preterm birth and PROM testing solutions facilitate early clinical intervention, planning, and management of preterm delivery risks to improve maternal and fetal health outcomes. The market is driven by rising preterm births, advancements in prenatal diagnostics, and increasing demand for personalized prenatal screening
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




