Pharmacogenetic Testing Market Size and Trends
The global pharmacogenetic testing market is estimated to be valued at USD 14.33 Bn in 2025 and is expected to reach USD 26.72 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market is expected to witness significant growth over the forecast period. This can be attributed to the rising application of pharmacogenetic testing across therapeutic areas such as oncology, neurology, and pain management. Growing need for precision medicine and rising focus on reducing trial and error in drug development will further stimulate market revenue through 2032.
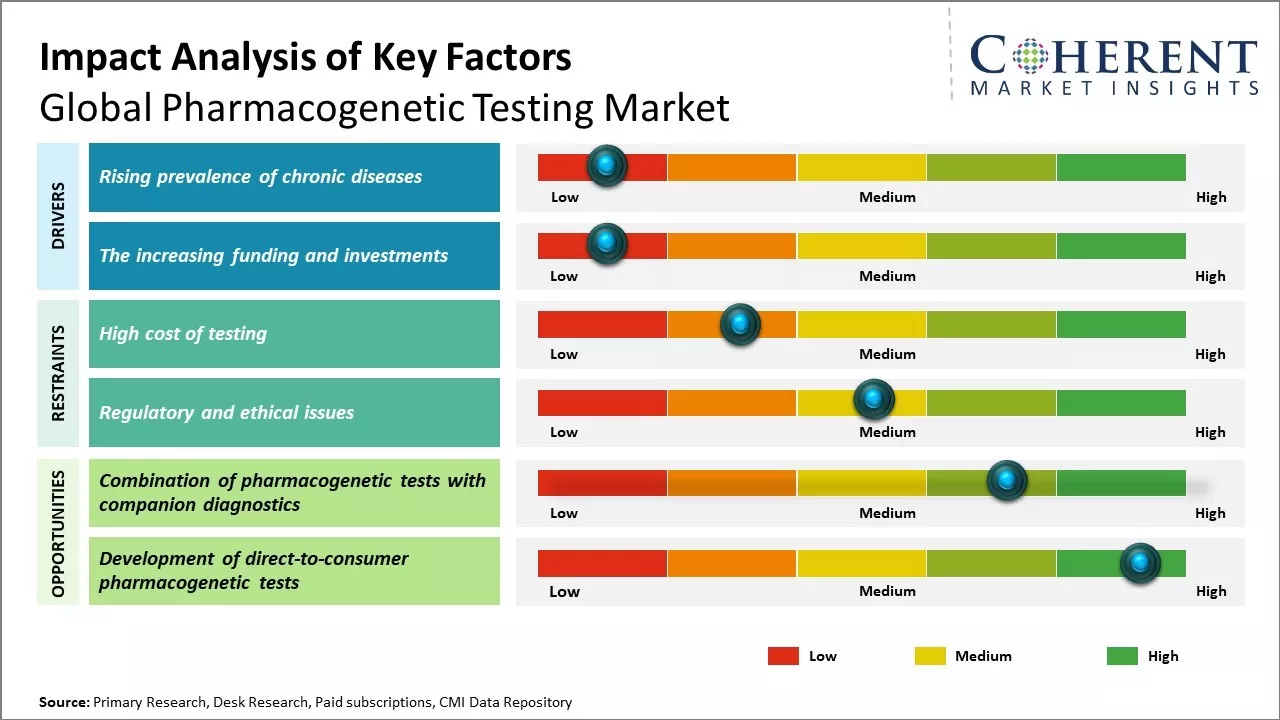
Rising prevalence of chronic diseases
The global burden of chronic diseases has been increasing significantly over the past few decades. Chronic diseases such as cancer, cardiovascular disease, diabetes, and neurological disorders are amongst the leading causes of mortality worldwide. It is estimated that over 50% of cancers are caused due to genetic and lifestyle factors. With advancements in genomics and pharmacogenomics, the role of DNA variations and mutations in disease etiology is being increasingly understood. Pharmacogenetic testing helps identify individual genetic variations that impact drug metabolism and response. This enables healthcare providers to prescribe safer and more effective medications tailored to the patient's genetic profile. With a rapidly growing chronic disease population worldwide, demand for more personalized treatment approaches is on the rise. Pharmacogenetic testing can help optimize drug therapy for chronic diseases and reduce trial-and-error based prescribing practices. This is expected to drive greater adoption of pharmacogenetic testing services globally in the coming years.
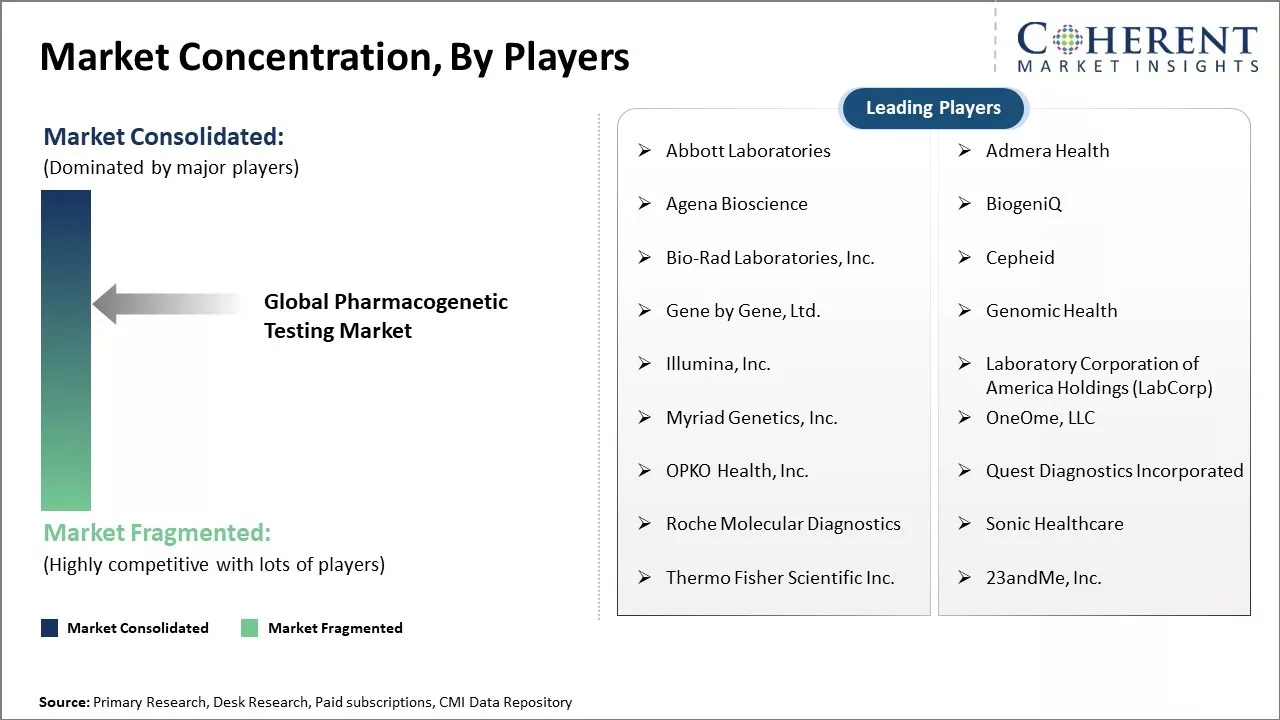
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
The increasing funding and investments
The increasing funding and investments directed towards DNA sequencing programs globally is significantly driving the growth of the pharmacogenetic testing market. Various governments and non-profit organizations are investing heavily to support DNA sequencing initiatives that study genetic variations and understand how genes impact an individual's response to medications. For instance, the National Human Genome Research Institute, a part of the United States National Institutes of Health, invested over US$806 million in 2020 towards Genome projects alone. Such large investments are helping accelerate DNA sequencing research. Several clinical research studies utilizing DNA sequencing are helping pharmacogenetic testing gain more prominence. DNA sequencing studies are helping identify genetic markers that influence drug metabolism and effectiveness. They are correlating genetic variants to adverse drug reactions. For instance, according to a May 2022 report from the NIH of the U.S., spending on cancer genomics research has increased notably, rising from USD 1,160 million in 2021 to USD 1,220 million. This significant investment in genomics and personalized medicine is expected to positively influence the growth of the pharmacogenomics market. Pharmacogenomics plays a crucial role in cancer treatment, enhancing patient survival rates while also reducing unnecessary costs associated with ineffective treatments.
Key Takeaways from Analyst
The global pharmacogenetic testing market promises strong growth in the coming years. Major drivers for the market include the rising demand for precision medicine and the increasing focus on pharmacogenetic testing to optimize drug efficacy and safety. As healthcare moves towards more tailored medical solutions based on an individual's genetic makeup, pharmacogenetic testing will play a key role in determining the right drug and dosage for patients.
North America currently dominates the global market due to supportive regulatory policies and approval environment for innovative diagnostic tests in the U.S. However, Asia Pacific is expected to emerge as the fastest growing regional market. This can be attributed to rising collaborations between global pharma companies and testing providers with local players in countries like China and India. Economic expansion in these populous nations also means a large patient pool for pharmacogenetic services.
While the need for such testing services is growing rapidly, there are also certain challenges holding back market growth. High costs associated with pharmacogenomic tests and lack of adequate reimbursement models remain key restraints, especially in developing markets. Meanwhile, inconsistent guidelines and clinical validity of many tests also restrain wider clinical adoption and utilization.
Market Challenges: High cost of testing
The high cost of pharmacogenetic testing is significantly restraining the global market's growth potential. Pharmacogenetic testing helps determine how patients will respond to specific medicines based on their genetic makeup. However, these tests can be quite expensive, ranging from hundreds to thousands of dollars per patient. This high cost puts the tests out of reach for many individuals and healthcare systems. The expenses associated with pharmacogenetic testing are burdensome for several reasons. Developing accurate and reliable genetic tests requires extensive research and development investments by laboratories. The process of analyzing a patient's DNA and matching it to various drug metabolisms and interactions is technically complex as well. Laboratories must employ specialized equipment, skilled technicians, and interpretive genetic counselors to perform these tests. All of these factors contribute to the high per-patient costs.
Market Opportunities: Combination of pharmacogenetic tests with companion diagnostics
The combination of pharmacogenetic testing with companion diagnostics presents a significant opportunity for the global pharmacogenetic testing market. Pharmacogenetic testing helps determine how an individual's genetics could influence their response to drugs by analyzing genetic variations. This allows physicians to select the most effective medicines and dosage for a patient's genetic makeup. Companion diagnostics are diagnostic tests developed alongside new drugs to identify the patients who will likely respond to a specific treatment. By combining pharmacogenetic testing with companion diagnostics, healthcare providers can gain invaluable insights into personalized treatment options for their patients. For example, companion diagnostic tests for certain cancer drugs can identify patients with specific genetic mutations or biomarkers that indicate they will clinically benefit from a targeted therapy. Meanwhile, pharmacogenetic testing can reveal other genetic factors that influence how a patient metabolizes and responds to that treatment. The complementary data from both tests empowers doctors to develop truly individualized treatment plans, maximizing drug efficacy and safety outcomes. Avoiding non-responsive treatments also has economic advantages in minimizing unnecessary healthcare expenditures.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
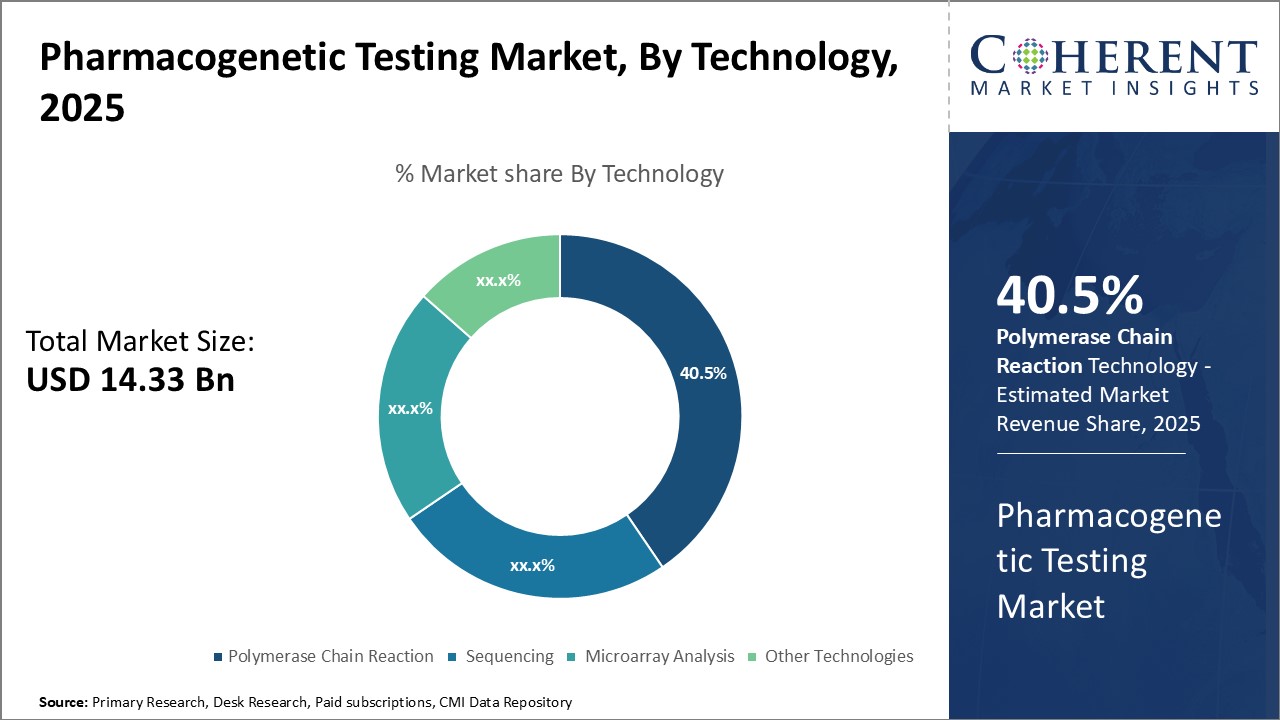
Insights By Technology - Advancements in PCR technology drive its leading share
In terms of technology, Polymerase Chain Reaction (PCR) is expected to contributes the highest share of the market, accounting for 40.1 of the market share in 2025 owing to the continuous advancements and improvements in PCR technology. PCR enables the rapid replication of specific DNA sequences which allows the detection of genetic mutations and variations. Traditional PCR technologies were expensive and labor-intensive. However, with ongoing R&D, the latest PCR platforms offer highly automated, easy-to-use and cost-effective solutions. Real-time quantitative PCR variants such as RT-qPCR have enabled high-throughput analysis and turned PCR into a reliable, accurate and affordable method for pharmacogenetic testing. Further innovations including high-resolution melting analysis, microfluidics and digital PCR have increased PCR's sensitivity and multiplexing capabilities. This has expanded its applications from academic research to clinical diagnostics. Hence, PCR continues to be the most widely used technique in pharmacogenetic tests due to ease of use, affordability, and constant technology upgrades which makes it preferred by healthcare providers and patients alike.
Insights By Application - Diverse applications in cancer management drive the oncology segment
In terms of application, oncology is expected to contribute the highest share of the market, accounting for 20.62% of the market share in 2025 owing to the diverse applications of pharmacogenetic testing in cancer management and personalized treatment. Genetic variations play a key role in variable drug response in cancer patients. Pharmacogenetic testing helps identify patients likely to respond or develop adverse reactions to certain chemotherapy drugs, targeted therapies, or immunotherapies. This allows doctors to select the most effective medication and avoid trial-and-error approaches. For example, testing for genetic mutations in EGFR, KRAS, BRAF, etc. guides choices of targeted drugs in lung and colorectal cancers. Similarly, testing for BRCA1/2 variants identifies patients susceptible to PARP inhibitors for ovarian cancer. Pharmacogenetic analysis of metabolic enzymes helps minimize toxicities from commonly used anti-cancer drugs like 5-fluorouracil, tamoxifen, and irinotecan. With the focus shifting to precision and personalized cancer care, pharmacogenetic testing is being widely adopted for optimizing cancer treatment outcomes as well as improving survival rates and quality of life for patients.
Insights By End User - Cost benefits drive hospital adoption of pharmacogenetic testing
In terms of end user, hospitals & clinics is expected to contribute the highest share of the market with 40.12 % in 2025 owing to the economic advantages of pharmacogenetic testing adoption. Incorporating pharmacogenetic testing promises considerable cost savings for hospitals from reduced trial-and-error prescribing, treatment failures, extended hospital stays, and adverse drug reactions. For example, warfarin sensitivity testing can help avoid serious bleeding risks which account for prolonged hospitalizations and increased medical expenses. Genetic screening prior to chemotherapy and target therapy selection can minimize hospital visits due to lack of response or toxicity issues. It also improves reimbursement through value-based payment models focusing on quality and effective patient outcomes. Significant R&D investment and technological developments have made tests more simple, accessible and affordable. This addresses the budget and resource constraints of hospitals especially in developing regions. Additionally, access to expertise and ease of reporting genetic results makes hospitals a suitable environment for incorporating pharmacogenetics into clinical workflows. Being the healthcare facilities with maximum patient throughput, hospitals can leverage the predicted high market demand as precision medicine rises.
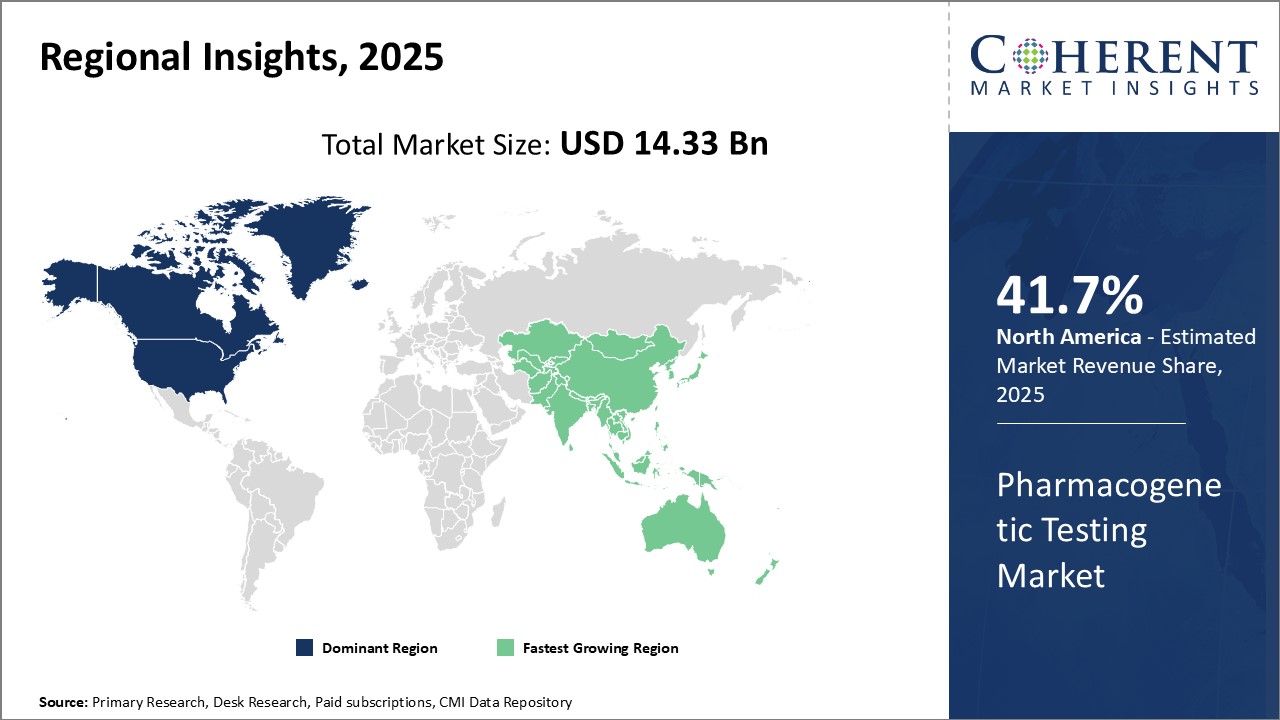
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America has emerged as the dominant region in the global pharmacogenetic testing market. The region is expected to account for a market share of 41.7% in 2025. The U.S. accounts for the largest share primarily due to the presence of key market players and availability of advanced healthcare facilities. The country has witnessed a significant increase in precision medicine initiatives by both public and private entities aiming to develop personalized treatment options for patients. Additionally, favorable regulatory policies have promoted the integration of pharmacogenetic testing to optimize drug prescription in the country. The growing demand for personalized drugs and diagnosis has encouraged pharmaceutical companies to invest heavily in R&D activities involving pharmacogenetics.
Asia Pacific is poised to be the fastest growing regional market during the forecast period. Rapid economic development across developing nations such as China and India has improved access to advanced healthcare facilities. The rising healthcare expenditure accompanied with growing geriatric population susceptible to chronic diseases has propelled the need for accurate diagnosis. Several local diagnostic players have entered partnerships with global leaders to provide affordable pharmacogenetic testing services. This has boosted their domestic market share. Simultaneously, concerns regarding drug efficiency and safety have driven increased adoption. Additionally, governments of various Asia Pacific countries have announced favorable regulatory guidelines and reimbursement policies to encourage precision medicine practices. This has spurred the integration of pharmacogenetic testing procedures among hospitals and clinics across the region.
Market Report Scope
Pharmacogenetic Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 14.33 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.3% | 2032 Value Projection: | USD 26.72 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott Laboratories, Admera Health, Agena Bioscience, BiogeniQ, Bio-Rad Laboratories, Inc., Cepheid, Gene by Gene, Ltd., Genomic Health, Illumina, Inc., Laboratory Corporation of America Holdings (LabCorp), Myriad Genetics, Inc., OneOme, LLC, OPKO Health, Inc., Quest Diagnostics Incorporated, Roche Molecular Diagnostics, Sonic Healthcare, Thermo Fisher Scientific Inc., and 23andMe, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Segmentation
- Technology Insights (Revenue, USD Bn, 2020 - 2032)
- Polymerase Chain Reaction (PCR)
- Sequencing
- Microarray Analysis
- Other Technologies
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Cardiology
- Gastroenterology
- Endocrinology
- Immunology & Hypersensitivity
- Gynecology
- Oncology
- Neurology
- Others
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals & Clinics
- Academic & Research Institutes
- Biopharmaceutical Companies
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Abbott Laboratories
- Admera Health
- Agena Bioscience
- BiogeniQ
- Bio-Rad Laboratories, Inc.
- Cepheid
- Gene by Gene, Ltd.
- Genomic Health
- Illumina, Inc.
- Laboratory Corporation of America Holdings (LabCorp)
- Myriad Genetics, Inc.
- OneOme, LLC
- OPKO Health, Inc.
- Quest Diagnostics Incorporated
- Roche Molecular Diagnostics
- Sonic Healthcare
- Thermo Fisher Scientific Inc.
- 23andMe, Inc.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
