Pharmaceutical Stability And Storage Services Market Size and Trends
Global pharmaceutical stability and storage services market is estimated to be valued at USD 3.37 Bn in 2025 and is expected to reach USD 5.07 Bn by 2032, growing at a compound annual growth rate (CAGR) of 6.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market is expected to witness positive growth during the forecast period. Increasing regulatory stringency regarding stability testing and storage compliance is prompting pharmaceutical companies to outsource these services to specialized providers. Furthermore, growing complexity of drug formulations is boosting the need for sophisticated stability testing protocols and specialized storage facilities. Market players are increasingly offering integrated stability testing and storage services to help sponsors comply with various regulatory requirements more efficiently. Furthermore, the COVID-19 pandemic has increased demand for temperature-controlled storage from vaccine manufacturers, which is accentuating the market growth. However, high service costs may hinder the growth of small and mid-sized pharmaceutical companies to some extent.
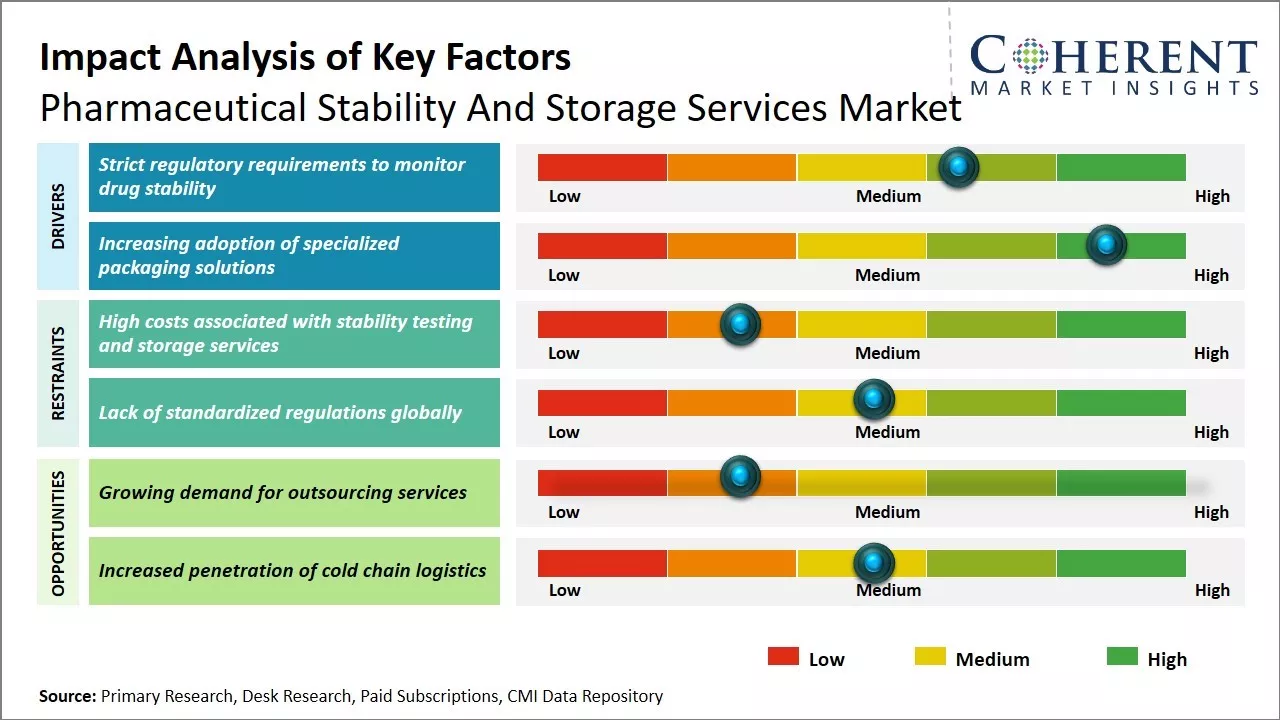
Strict regulatory requirements to monitor drug stability
Pharmaceutical manufacturers around the globe are increasingly focused on ensuring the quality, potency and safety of their drugs throughout the supply chain. Regulatory authorities like the U.S. FDA have stringent guidelines for drug stability testing and require manufacturers to monitor drug samples periodically post approval as well to identify any degradation in drug quality. Stability testing helps to determine the shelf-life and appropriate storage conditions of drugs. It also helps manufacturers comply with cGMP and meet the standards specified in product labels.
With an expanding pharmaceutical portfolio and greater oversight on product quality, it has become imperative for companies to outsource stability testing & monitoring to specialized service providers. These providers offer advanced analytical testing, long-term stability studies, stress testing and specialized storage facilities across temperature zones to accurately simulate supply chain conditions. Their dedicated stability programs help pharmaceutical companies stay compliant with regulatory norms and timely address any deviations observed during monitoring.
Market Concentration and Competitive Landscape

Get actionable strategies to beat competition: Download Free Sample
Increasing adoption of specialized packaging solutionsPharmaceutical packaging plays a vital role in protecting drugs from external contaminants and preventing any chemical or physical degradation during transportation and storage. With global supply chains expanding, there is a growing emphasis on developing innovative packaging technologies that can withstand varied climate conditions and ensure drug stability for longer durations. Pharmaceutical manufacturers are actively partnering with packaging service providers to implement specialized solutions like moisture-tight seals, oxygen absorbers, tracking devices and thermal-protective containers. Some key trends include greater use of materials like glass and laminates that offer premium barrier protection from air, moisture and light.
To learn more about this report, Download Free Sample
Market Challenges – High costs associated with stability testing and storage servicesThe high costs associated with stability testing and storage services impose a substantial restraint on the growth of the pharmaceutical stability and storage services market. Conducting stability testing over the long term to confirm the shelf life of drugs and maintain stringent quality standards throughout mandated storage periods requires immense financial investments, thereby, hampering the market development over the forecasted period.
Market Opportunities – Growing demand for outsourcing services
The market witnesses numerous opportunities for growth as pharmaceutical companies increasingly turn to outsourcing partners to offload stability testing responsibilities and ensure regulatory compliance. New development in digital monitoring technologies allow for round-the-clock remote supervision of storage facilities. There is also demand for integrated testing plans that combine accelerated, stress, and real-time studies into comprehensive programs. Partnerships between drug makers and contract service providers have expanded to encompass full lifecycle management.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
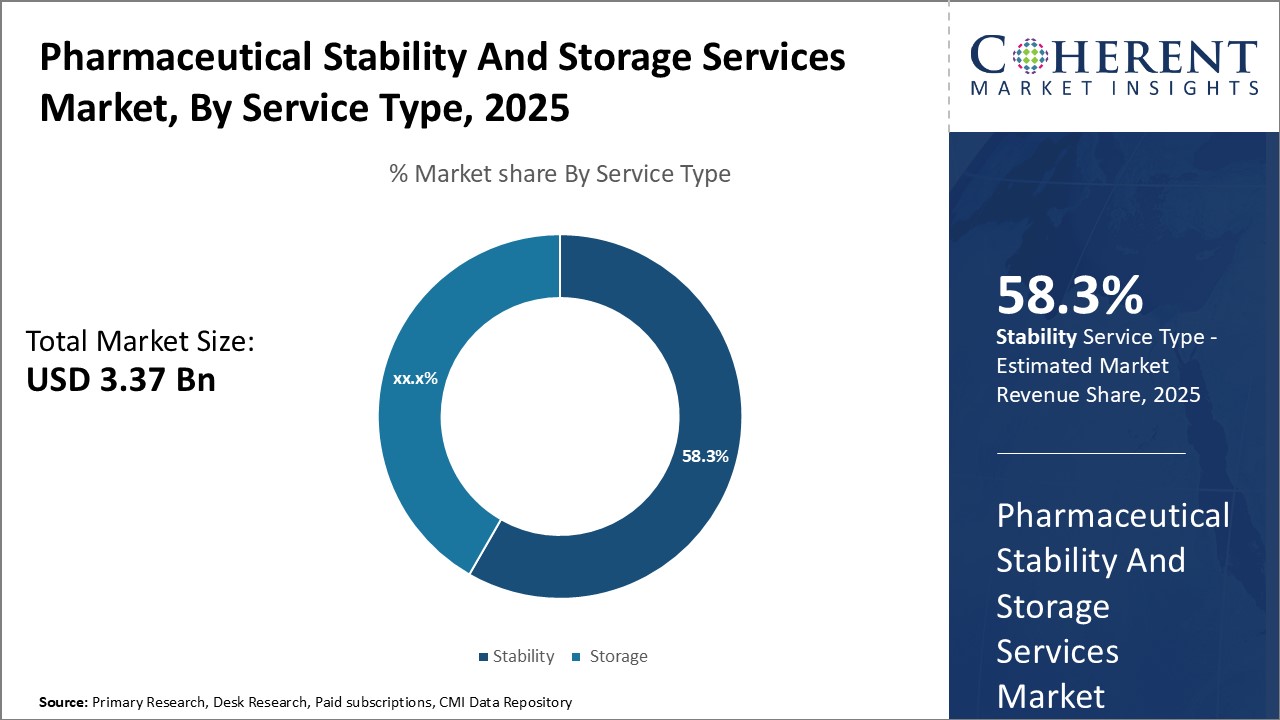
Insights, By Service Type: Growing demand for stability testing servicesThe service type segment includes stability and storage. In terms of service type, stability segment contributes the highest share of the market owing to increasing stringent stability-testing norms and is anticipated to secure 58.3% market share in 2025. Regulatory mandates require drugs to undergo rigorous stability testing throughout their shelf life to ensure consistent performance under different climatic conditions. This has significantly boosted demand for stability testing services from pharmaceutical manufacturers. Emerging biologics and complex drugs have further increased stability-testing needs due to greater susceptibility to physical and chemical degradation during storage and distribution. Growing requirements to study drug stability across container closure systems have also supported segment growth. Furthermore, the capability of stability testing to verify expiry dates and guarantee batch integrity throughout distribution networks makes it crucial for compliance with current good manufacturing practices.
Insights, By Product Type: Rising demand for oral solid dosage drugs boosts tablets segment growth
The product type segment includes tablets, capsules, injectable solutions, oral suspensions, combination products, and others. Among the product type, tablets segment is anticipated to have 28.3% of the market share in 2025. Tablets account for the majority of global drug production volume due to convenience of administration and favorable stability profile. The large-scale manufacture and global distribution of tablets necessitates optimized cold chain management and storage infrastructure capable of handling bulk inventories. Additionally, regulatory needs to securely store retained drug samples from clinical trials and post-marketing surveillance activities have further boosted demand. The capability of centralized depots to meet global distribution requirements while maintaining drug quality makes professional storage services indispensable for the thriving tablet drugs market.
Insights, By End User: Outsourcing drives biopharmaceutical companies segment
The end user segment includes biopharmaceutical companies, CMO, CRO, and others. Among end user, biopharmaceutical companies segment is anticipated to hold 35.4% of the market share in 2024 as large biologic drug producers increasingly rely on capable outsourcing partners for stability and storage services. High development costs of biologics encourage outsourcing non-core operations to optimize capital expenditures. Furthermore, CROs/CMOs offer biopharmaceutical companies access to state-of-the-art stability chambers and temperature-controlled warehouses alongside scientific expertise. Their scale allows for cost-effective validation of complex biologic storage conditions on a large commercial scale. The expanding pipeline of biologics therefore benefits associated contract service providers. Furthermore, tight regulatory quality requirements make outsourcing to specialize service providers an attractive proposition for ensuring compliance.
Regional Insights
Need a Different Region or Segment? Download Free Sample
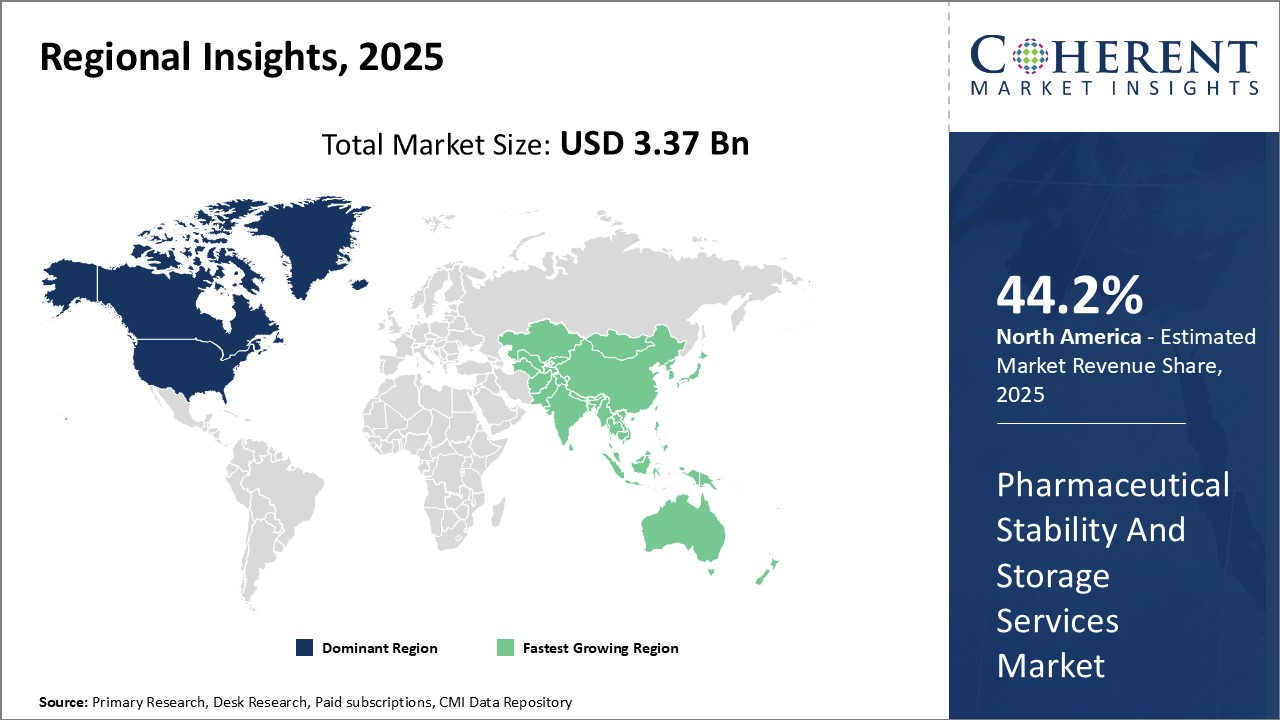
North America continues to dominate the global pharmaceutical stability and storage services market and is anticipated to hold 44.2% market share in 2025. This can be attributed to strong presence of leading pharmaceutical companies in the region with large R&D budgets. With stringent regulatory guidelines around drug stability testing and data requirements from the U.S. FDA, pharmaceutical companies rely heavily on outsourcing such services to ensure compliance. North America has seen major infrastructure investments by key players to establish state-of-the-art stability storage facilities. This has ensured easy access to on-demand services for pharmaceutical firms.
The Asia Pacific region is poised to be the fastest growing market for pharmaceutical stability and storage services over the forecasted period. Given the cost advantages and policy support for generic drug manufacturing, Asia Pacific has emerged as the hub for generic drug exports worldwide. However, testing and data requirements to comply with international standards remains a challenge for domestic firms. This has increased reliance on specialized stability testing providers. Organizations are also outsourcing drug sample storage to third parties with the best-in-class controlled temperature and humidity storage capabilities. The region benefits from lower operating costs for service players compared to mature markets. This is expected to further boost adoption of stability and storage services among pharmaceutical manufacturers expanding in Asia Pacific.
Market Report Scope
Pharmaceutical Stability And Storage Services Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.37 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.0% | 2032 Value Projection: | USD 5.07 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Intertek Group plc, Eurofins Scientific, SGS Société Générale de Surveillance SA., Q Laboratories, BioLife Solutions Inc., Cencora, Inc., Alloga, PCI Pharma Services, Pharmaserv GmbH, Catalent, Inc, Almac Group, Charles River Laboratories, Lucideon, Alcami Corporation, Element Materials Technology, Nelson Laboratories, LLC, ALS |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Segmentation
- Service Type Insights (Revenue, USD BN, 2020 - 2032)
- Stability
- Accelerated Stability Testing
- Photostability
- Long Term Drug Stability Testing
- Forced Degradation Testing
- Others
- Storage
- Cold Storage
- Controlled 15-25°C
- Refrigerated 2-8°C
- Frozen -24°C
- Cryogenic -170°C
- Non - Cold Storage
- Cold Storage
- Stability
- Product Type Insights (Revenue, USD BN, 2020 - 2032)
- Tablets
- Capsules
- Injectable Solutions
- Oral Suspensions
- Combination Products
- Others
- End User Insights (Revenue, USD BN, 2020 - 2032)
- Biopharmaceutical Companies
- CMO
- CRO
- Others
- Regional Insights (Revenue, USD BN, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Intertek Group plc
- Eurofins Scientific
- SGS Société Générale de Surveillance SA.
- Q Laboratories
- BioLife Solutions Inc.
- Cencora, Inc.
- Alloga
- PCI Pharma Services
- Pharmaserv GmbH
- Catalent, Inc
- Almac Group
- Charles River Laboratories
- Lucideon
- Alcami Corporation
- Element Materials Technology
- Nelson Laboratories, LLC
- ALS
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
