Pharmaceutical Fine Chemicals Market Size And Share Analysis - Growth Trends And Forecasts (2025-2032)
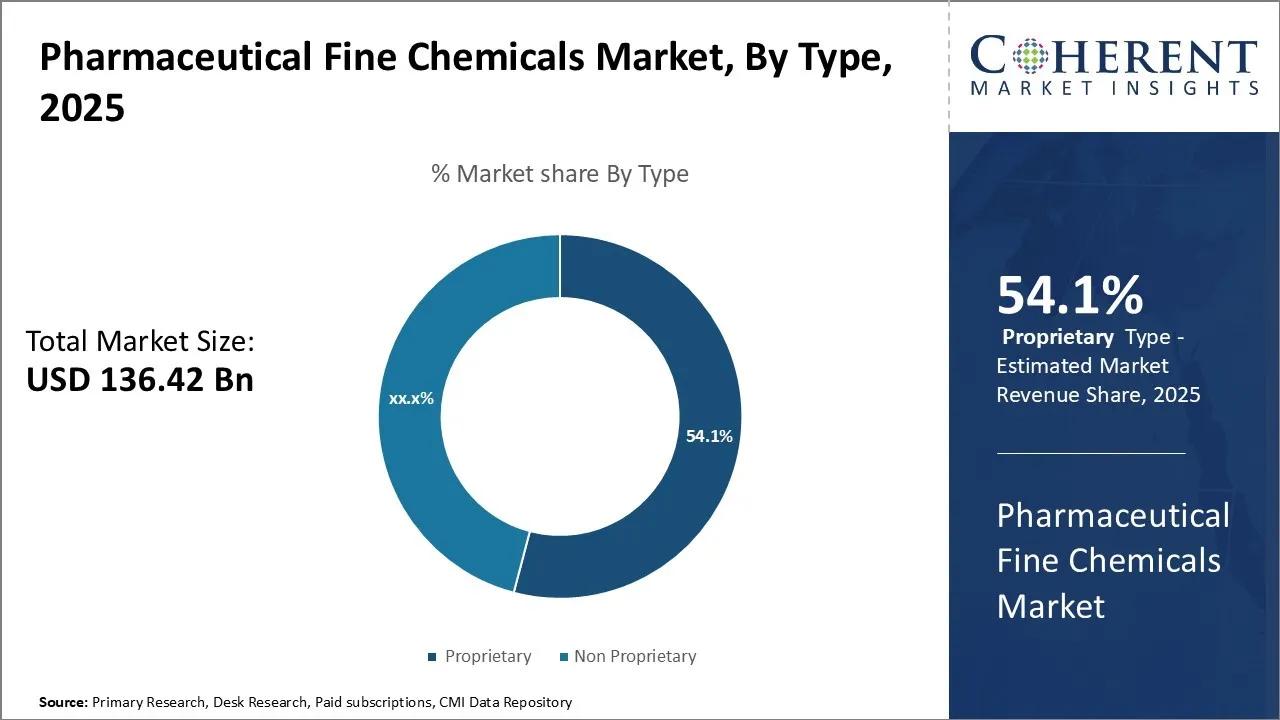
The pharmaceutical fine chemicals market is projected to exhibit substantial growth, increasing from USD 136.42 Bn in 2025 to an estimated USD 235.32 Bn by 2032 this growth is anticipated to be driven by a notable CAGR of 8.1% during the period of 2025–2032.
Key Takeaways
- Based on Type, the Proprietary segment is projected to hold a 54.1% of the market share in 2025, due to the growing need for customized, high-purity active pharmaceutical ingredients (APIs) and intermediates tailored for branded drugs.
- Based on Product, the Active Ingredient segment is estimated to account for the largest share of the market in 2025, on account of the growing need for high-purity active pharmaceutical ingredients (APIs) in drug manufacturing.
- Based on Application, the Oncological segment is expected to capture the highest share of the market in 2025 due to the rising global incidence of cancer and the increasing adoption of targeted and personalized therapies.
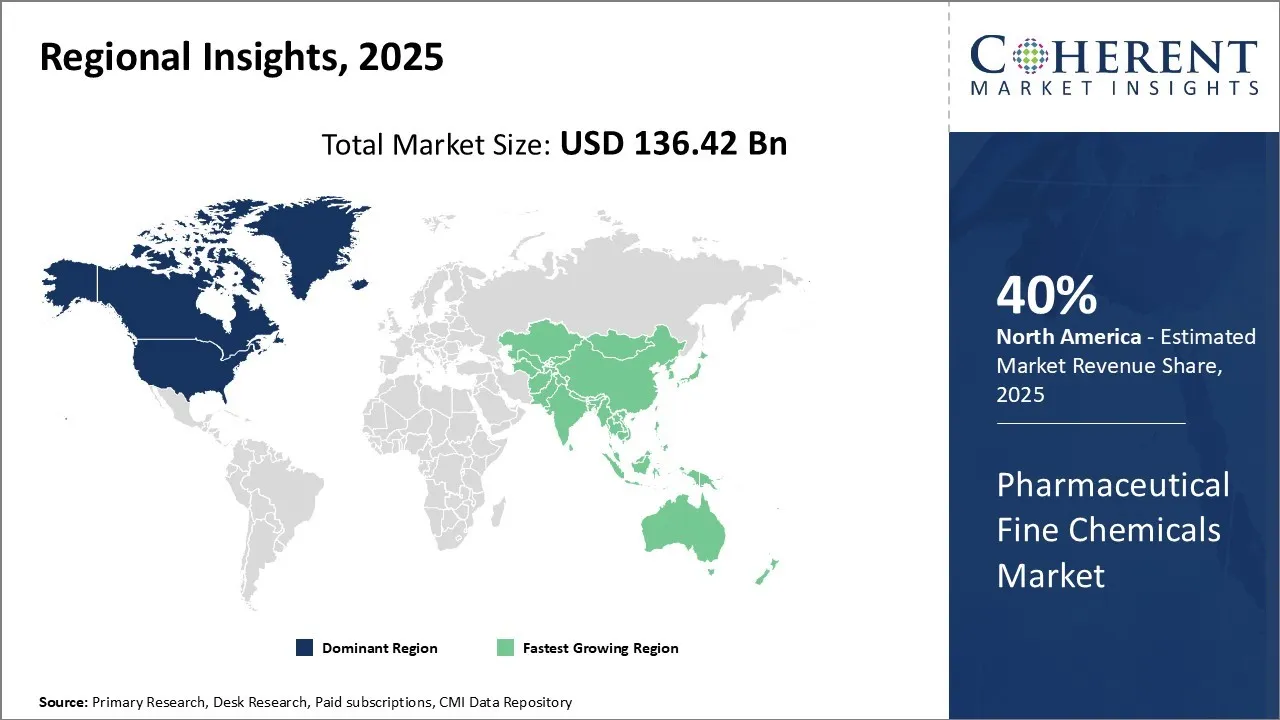
- Based on Region, North America is set to lead the global pharmaceutical fine chemicals market in 2025 with a 40% share. While, Asia Pacific, holding an estimated share of 30% in 2025, and it is expected to be the fastest growing region.
Market Overview
The pharmaceutical fine chemicals market growth is witnessing steady growth, driven by increasing demand for high-purity active pharmaceutical ingredients (APIs) and intermediates used in drug manufacturing. Rising prevalence of chronic diseases, expansion of the global pharmaceutical industry, and growing emphasis on specialty and complex therapeutics are fueling market adoption. Continuous innovations in synthesis technologies, contract manufacturing, and regulatory compliance further enhance production efficiency. The demand for cost-effective and high-quality chemical intermediates for generic and branded drugs is also supporting growth, making pharmaceutical fine chemicals market demand increasingly robust across developed and emerging regions.
Current Events and their Impact on the Pharmaceutical Fine Chemicals Market
|
Current Event |
Description and its Impact |
|
Geopolitical Tensions and Supply Chain Vulnerabilities |
|
|
Technological Transformation and Digital Integration |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
End User Feedback and Unmet Needs of the Pharmaceutical Fine Chemicals Market
End-User Feedback
- High-Quality Standards Required – Pharmaceutical companies emphasize the need for consistent, impurity-free, and regulatory-compliant intermediates and APIs to ensure product safety and efficacy.
- Reliability of Supply – Users value suppliers that can provide uninterrupted and timely delivery, especially for critical drug intermediates in generics and biosimilars.
- Cost-Effectiveness – End users seek competitive pricing without compromising quality, particularly in emerging markets where budget constraints are significant.
- Technical Support and Customization – Companies appreciate suppliers who offer technical expertise, customized synthesis routes, and scalable production solutions.
Unmet Needs
- Complex Molecule Synthesis – Growing demand for advanced biologics, biosimilars, and novel small molecules highlights the need for specialized fine chemicals that are difficult to synthesize in-house.
- Faster Development Timelines – Pharmaceutical firms require suppliers that can accelerate production cycles to match the speed of drug discovery and clinical trials.
- Sustainable and Green Chemistry Practices – There is increasing pressure for eco-friendly production processes that reduce waste and energy consumption.
- Global Regulatory Compliance – Manufacturers need suppliers capable of meeting diverse international standards, including FDA, EMA, and ICH guidelines.
Pharmaceutical Fine Chemicals Market Insights, By Type: Proprietary leads the market Owing to the Growing Need for Customized, High Purity APIs and Intermediates
In terms of type, the proprietary segment is expected to contribute the highest share of 54.1% in the market in 2025, due to the growing need for customized, high-purity active pharmaceutical ingredients (APIs) and intermediates tailored for branded drugs. Pharmaceutical companies prefer proprietary chemicals to maintain competitive advantage, patent protection, and product differentiation. Additionally, the rising focus on complex therapeutics, biologics, and specialty drugs requires highly specific and controlled chemical compositions, which proprietary fine chemicals provide. Regulatory compliance, quality assurance, and supply chain reliability further drive the adoption of proprietary products over standard or generic alternatives.
For instance, in July 2025, Udaipur-based Macsen Labs has filed a provisional patent for its proprietary synthesis process of Prussian White, a high-performance cathode material for sodium-ion batteries. This breakthrough follows the company's unexpected discovery while researching Prussian Blue for pharmaceutical applications. The synthesized Prussian White exhibits an energy density exceeding 150 mAh/g, comparable to Lithium Iron Phosphate (LFP), and offers excellent stability and rapid sodium-ion mobility due to its open crystalline structure, which is further propelling the pharmaceuticals fine chemicals market share.
Pharmaceutical Fine Chemicals Market Insights, By Product: Active Ingredient is Demanding Due to Growing Need for High-Purity APIs
In terms of product, the active ingredient segment is expected to capture the largest share of the market in 2025, due to the growing need for high-purity active pharmaceutical ingredients (APIs) in drug manufacturing. Rising prevalence of chronic and complex diseases, including cancer, cardiovascular, and neurological disorders, is driving demand for effective and targeted therapies. Additionally, the expansion of generic drugs, biologics, and specialty medicines requires reliable, high-quality active ingredients to ensure efficacy and safety. Increasing investments in pharmaceutical R&D, along with stringent regulatory requirements for drug formulations, further fuel the adoption of high-grade active ingredients in global pharmaceutical manufacturing.
For instance, in March 2025, Allchem Lifescience, a Gujarat-based pharmaceutical firm specializing in active pharmaceutical ingredients (API) intermediates and specialty chemicals, has filed for an initial public offering (IPO) worth ₹905 crore with the Securities and Exchange Board of India (SEBI). The offering includes a fresh issue of ₹190 crore and an offer-for-sale (OFS) of up to 7.15 million equity shares, amounting to ₹715 crore.
Pharmaceutical Fine Chemicals Market Insights, By Application: Oncological Dominance is Driven by Rising Global Cancer Incidence
In terms of application, the oncological segment holds the highest share of the market in 2025, due to the rising global incidence of cancer and the increasing adoption of targeted and personalized therapies. Advanced anticancer drugs require high-purity active pharmaceutical ingredients (APIs) and complex chemical intermediates, which are produced by fine chemicals manufacturers. Continuous research and development in oncology, coupled with the growing focus on biologics, immunotherapies, and combination therapies, further drive demand. Additionally, regulatory approvals for new cancer treatments and expanding healthcare access in emerging markets are contributing to the sustained growth of oncological applications within the market.
For instance, in May 2025, Skyepharma has achieved significant milestones at its MMPP (Medicines to be Handled with Particular Care) facility in France, dedicated to high-potency oral oncology drug production. The 450 m² GMP-compliant unit, operational for six months, is designed to handle cytotoxic and cytostatic compounds up to OEB4/5+ safety standards. The facility has secured four industrial partnerships, created 15 specialized jobs, and is projected to generate €10 million in annual revenue from oncology-related services. With a manufacturing capacity exceeding 80 million tablets and 200 million capsules annually.
Regional Insights
To learn more about this report, Download Free Sample
North America Pharmaceutical Fine Chemicals Market Analysis & Trends
North America has dominated the global pharmaceutical fine chemicals market over the past few decades. The market in North America is expected to be the fastest growing market for pharmaceutical fine chemicals, with a CAGR of over 40% during the forecast period. The region enjoys strong industry presence from leading global pharmaceutical manufacturers as most major players have their headquarters located in the U.S. This has led to well-established supply chains within the region to cater to the constant demand from domestic producers.
For instance, in June 2025, Lubrizol expanded its distribution agreement with IMCD to meet the growing demand for medical solutions in the United States and Canada. This collaboration extends their successful medical thermoplastic polyurethane (TPU) partnership from Europe to North America. IMCD will leverage its expertise in medical-grade polymers to enhance market penetration, manage inventory efficiently, and strengthen customer relationships. Lubrizol offers a comprehensive range of medical-grade TPU solutions, while IMCD provides specialized distribution and formulation services for specialty chemicals and ingredients.
Asia Pacific Pharmaceutical Fine Chemicals Market Analysis & Trends
Asia Pacific region has emerged as the fastest growing market for pharmaceutical fine chemicals in recent times, with a CAGR of over 30% during the forecast period. The growth of the market in the Asia Pacific region can be attributed to rapid industrialization and increasing government investments in healthcare sectors across developing countries, thus boosting the regional consumption. Countries like India and China have especially strengthened their domestic industries to lower import dependence and benefit from cost competitiveness. Their large and growing population bases have attracted numerous international pharmaceutical companies to establish local manufacturing sites through joint ventures or acquisitions.
For instance, in April 2025, Barentz, a global specialty ingredients provider, entered exclusive discussions to acquire 100% equity of Fengli Group, a leading distributor of pharmaceutical excipients and active ingredients in China. The acquisition aims to strengthen Barentz's presence in the Asia-Pacific pharmaceutical market by leveraging Fengli's established network and technical expertise.
Pharmaceutical Fine Chemicals Market Outlook Country-Wise
The U.S. Pharmaceutical Fine Chemicals Market Trends
The pharmaceutical fine chemicals market in the U.S. is witnessing strong demand due to the country’s well-established pharmaceutical and biotechnology sectors. High prevalence of chronic and complex diseases, such as cancer, cardiovascular disorders, and diabetes, drives the need for advanced active pharmaceutical ingredients (APIs) and chemical intermediates. Additionally, ongoing research and development, continuous adoption of innovative drug formulations, and stringent quality and regulatory standards boost production and consumption of fine chemicals. The growth of contract manufacturing organizations (CMOs) and increasing focus on specialty and high-purity chemicals further support the pharmaceutical fine chemicals market growth in the U.S.
For instance, in June 2025, BASF inaugurated a new Good Manufacturing Practice (GMP) Solution Center in Wyandotte, Michigan, reinforcing its commitment to the biopharma and pharmaceutical ingredients sectors. This state-of-the-art facility enhances BASF’s existing network of poloxamer sites and introduces advanced clean room packaging and high-sensitivity analytical testing capabilities.
China Pharmaceutical Fine Chemicals Market Trends
The pharmaceutical fine chemicals market in China is witnessing strong demand due to the country’s position as a global hub for active pharmaceutical ingredient (API) production and contract manufacturing. Rapid growth in the domestic pharmaceutical industry, rising prevalence of chronic and lifestyle-related diseases, and increasing healthcare expenditure are driving the consumption of high-purity intermediates and fine chemicals. Additionally, government initiatives supporting local drug manufacturing, research and development, and export of APIs are boosting market activity. The presence of well-established chemical manufacturing infrastructure and skilled workforce further reinforces China’s pivotal role in the pharmaceutical fine chemicals market.
For instance, in August 2024, Wanhua Chemical inaugurated the world's largest single-unit citral production facility in Yantai, China, with an annual capacity of 48,000 tonnes. Citral, a vital fine chemical used in fragrances, flavors, and nutritional products, is known for its complex production process and stringent technical requirements.
Pharmaceutical Fine Chemicals Market Drivers
- Rising Demand for Biosimilars
The development of biosimilars is creating significant opportunities for pharmaceutical fine chemical manufacturers. As major biologics go off-patent, biosimilars offer lower-cost alternatives, driving demand for specialized fine chemicals used in complex multi-step synthesis of biomolecules. Investments by global pharma companies, regulatory support, and growing adoption in emerging Asian markets are further boosting demand. Fine chemical suppliers play a critical role by providing high-purity bio-intermediates, enabling manufacturers to meet quality and compliance standards. Overall, the expanding biosimilars market is motivating producers to enhance and diversify their product portfolios to capitalize on this growing segment.
Pharmaceutical Fine Chemicals Market Opportunities
- Outsourcing of fine chemicals production
Outsourcing fine chemical production is emerging as a significant growth driver in the pharmaceutical fine chemicals market forecast. As drug development becomes increasingly complex, pharmaceutical companies are outsourcing non-core activities to contract manufacturing organizations (CMOs) to focus on discovery, clinical trials, and marketing. CMOs offer specialized facilities, cost-effective production of intermediates and APIs, and adherence to strict quality standards. This approach improves economies of scale, accelerates therapy development, and ensures regulatory compliance. As pharmaceutical R&D continues to advance, outsourcing fine chemical synthesis is expected to expand globally, creating substantial opportunities for market growth.
Market Report Scope
Pharmaceutical Fine Chemicals Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 136.42 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.1% | 2032 Value Projection: | USD 235.32 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Denisco, Albemarle Corporation, Kenko Corporation, GRACE, CHEMADA, JMP Statistical Discovery LLC., Pfizer Inc. and GSK plc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Analyst Opinion (Expert Opinion)
The Pharmaceutical Fine Chemicals Market is undergoing a pivotal transformation, driven by escalating demand for high-quality Active Pharmaceutical Ingredients (APIs), stringent regulatory standards, and the increasing complexity of drug formulations. This sector's evolution is not merely a reflection of growth but a response to the industry's pressing need for precision, safety, and scalability.
The market's trajectory is significantly influenced by the growing prevalence of chronic diseases such as cancer, diabetes, and neurological disorders. These conditions necessitate the development of specialized medications, thereby propelling the demand for fine chemicals. For instance, the rise in oncology therapies has led to an increased need for complex intermediates and advanced building blocks, which are essential for the synthesis of targeted therapies.
Furthermore, the shift towards personalized medicine is reshaping the landscape. Tailored treatments require bespoke chemical solutions, fostering innovation in the synthesis of APIs that cater to individual genetic profiles. This trend underscores the necessity for manufacturers to invest in research and development to stay competitive.
Regulatory compliance remains a cornerstone of the pharmaceutical fine chemicals sector. The implementation of stringent guidelines by authorities such as the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) ensures the safety and efficacy of pharmaceutical products. However, these regulations also pose challenges, including the need for continuous monitoring, documentation, and adherence to Good Manufacturing Practices (GMP). Companies must navigate these complexities to maintain market access and avoid costly penalties.
Recent Developments
- In August 2025, SCHOTT inaugurated the first local production of high-precision glass tubing for syringes and cartridges in India, at its Jambusar facility in Gujarat. This expansion addresses the rising demand for GLP-1-based injectables like semaglutide, used in diabetes and weight management The move aligns with India's 'Make in India' initiative, enhancing the nation's pharmaceutical self-reliance. Leveraging technology transferred from SCHOTT's German operations, the facility aims to meet the growing need for biologics administered via pre-filled syringes and cartridges.
- In March 2025, Amaran Biotech, a subsidiary of OBI Pharma, signed a Memorandum of Understanding (MOU) with Japan's Nippon Fine Chemical Co., Ltd. and its Taiwanese affiliate, Zillion Fine Chemicals International Co., Ltd. The collaboration aims to advance the contract development and manufacturing organization (CDMO) services for nanoparticle-based drugs, including liposomes and lipid nanoparticles (LNPs).
- In October 2024, Colorcon and LOTTE Fine Chemical have announced a global partnership to enhance pharmaceutical and dietary supplement formulations. Colorcon will exclusively represent LOTTE's AnyCoat-C® and AnyCoat-P® Hypromellose polymers. These products are integral to solution/suspension, controlled-release, immediate-release, and enteric-coated drug formulations. This strategic alliance aims to accelerate formulation development and meet the growing demand for plant-based pharmaceutical materials.
Market Segmentation
- Pharmaceutical Fine Chemicals Market, By Type
- Proprietary
- Non-Proprietary
- Pharmaceutical Fine Chemicals Market, By Product
- Basic Building Blocks
- Advanced Intermediates
- Active Ingredients
- Pharmaceutical Fine Chemicals Market, By Application
- Cardiovascular
- Neurological
- Oncological
- Infectious Diseases
- Metabolic System
- Diabetes
- Respiratory
- Gastrointestinal
- Musculoskeletal
- Others
- Pharmaceutical Fine Chemicals Market, By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Top companies in the pharmaceutical fine chemicals market
- Denisco
- Albemarle Corporation
- Kenko Corporation
- GRACE
- CHEMADA
- JMP Statistical Discovery LLC.
- Pfizer Inc.
- GSK plc.
Sources
Primary Research Interviews from the following stakeholders
Stakeholders
Interviews with pharmaceutical fine chemical manufacturers, contract manufacturing organizations (CMOs), active pharmaceutical ingredient (API) producers, chemical distributors, procurement heads, and R&D managers across leading global markets.
Specific Stakeholders
- R&D leads and process chemists at pharmaceutical companies
- Procurement and supply chain managers at API distributors and CMOs
- Facility and operations managers at chemical synthesis plants
- Regulatory affairs and quality assurance heads at pharmaceutical and fine chemical manufacturers
- Production managers at specialty chemical companies supplying intermediates
- Technical sales and product managers at chemical reagent suppliers
Databases
- World Trade Organization (WTO) Trade Statistics
- UN Comtrade Database
- U.S. Bureau of Economic Analysis
- Eurostat
- China Customs Statistics
- Korea Customs Service Data Portal
- Japan External Trade Organization (JETRO)
- Directorate General of Commercial Intelligence and Statistics (DGCIS), India
- Indian Ministry of Chemicals and Fertilizers
- European Chemicals Agency (ECHA) Database
Magazines
- Chemical & Engineering News (C&EN)
- Pharmaceutical Technology
- Chemical Week
- Fine Chemicals Magazine
- ICIS Chemical Business
- Pharma Manufacturing
Journals
- Journal of Pharmaceutical Sciences
- International Journal of Pharmaceutics
- Organic Process Research & Development
- European Journal of Pharmaceutical Sciences
- Journal of Medicinal Chemistry
- Drug Development and Industrial Pharmacy
Newspapers
- The Wall Street Journal – Health & Pharmaceuticals
- The Economic Times – Pharmaceuticals & Chemicals
- The Hindu Business Line – Pharma & Chemicals
- Financial Times – Healthcare & Life Sciences
- Nikkei Asia – Pharmaceutical & Chemical Supply Chain
- South China Morning Post – Health & Chemicals
Associations
- International Society for Pharmaceutical Engineering (ISPE)
- American Chemical Society (ACS)
- European Federation for Pharmaceutical Sciences (EUFEPS)
- Indian Pharmaceutical Association (IPA)
- Society of Chemical Industry (SCI)
- Association of Contract Research Organizations (ACRO)
Public Domain Sources
- U.S. Food and Drug Administration (FDA) – APIs & Drug Substances
- European Medicines Agency (EMA) – Regulatory & Market Data
- Ministry of Chemicals and Fertilizers, India
- National Institute of Standards and Technology (NIST), U.S.
- U.S. International Trade Commission (USITC)
- NITI Aayog – Pharmaceuticals & Chemicals Reports
- India Investment Grid – Chemicals & Pharma Sector
- EU Single Market Observatory – Chemical & Pharmaceutical Reports
- Reserve Bank of India (RBI) – Pharmaceutical & Chemical Sector
Proprietary Elements
- CMI Data Analytics Tool, and Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Yash Doshi is a Senior Management Consultant. He has 12+ years of experience in conducting research and handling consulting projects across verticals in APAC, EMEA, and the Americas.
He brings strong acumen in helping chemical companies navigate complex challenges and identify growth opportunities. He has deep expertise across the chemicals value chain, including commodity, specialty and fine chemicals, plastics and polymers, and petrochemicals. Yash is a sought-after speaker at industry conferences and contributes to various publications on topics related commodity, specialty and fine chemicals, plastics and polymers, and petrochemicals.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients