Pegfilgrastim Biosimilars Market Size and Trends
Global pegfilgrastim biosimilars market is estimated to be valued at USD 1.84 Bn in 2025 and is expected to reach USD 3.39 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.1% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market growth is driven by rising demand for cheaper biosimilar alternatives to the reference biologic drugs from patients struggling with healthcare costs. Biosimilars offer significant cost savings as compared to their reference biologics, and this boosts purchase of pegfilgrastim biosimilars by hospitals and cancer care centers. Rising incidence of cancer cases worldwide boosts the need for supportive care therapies such as pegfilgrastim. Moreover, the expiry of patents of major reference biologic drugs enable the commercialization of various novel biosimilars in the market. This can expand treatment access to more patients.
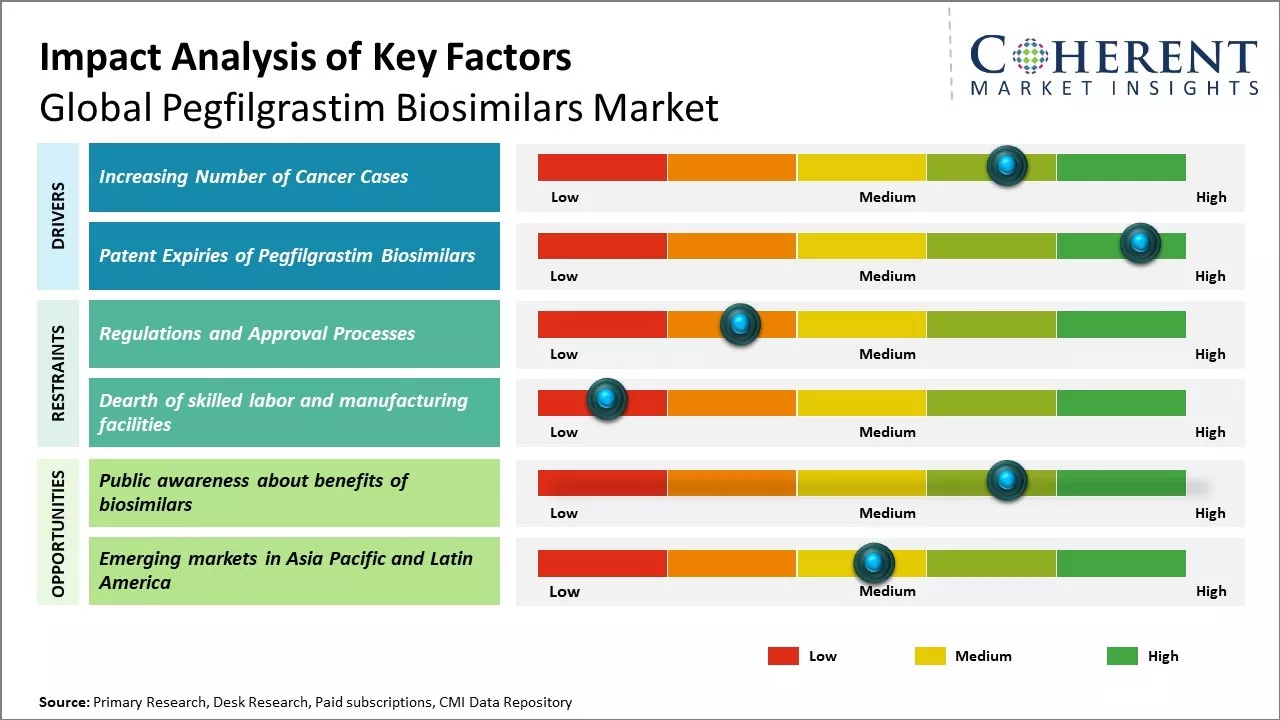
Increasing number of cancer cases
Rising incidence of cancer worldwide boosts demand for supportive cancer care therapies. According to WHO, cancer burden has nearly doubled since 2000 and is expected to increase in the near future. Cancer chemotherapy often results in severely low white blood cell count in patients, making them vulnerable to life-threatening infections. Pegfilgrastim is administered to patients undergoing chemotherapy to boost white blood cell count and reduce chances of infections. As more cancer patients opt for chemotherapy globally each year, there will be huge demand for pegfilgrastim to protect them from neutropenia.
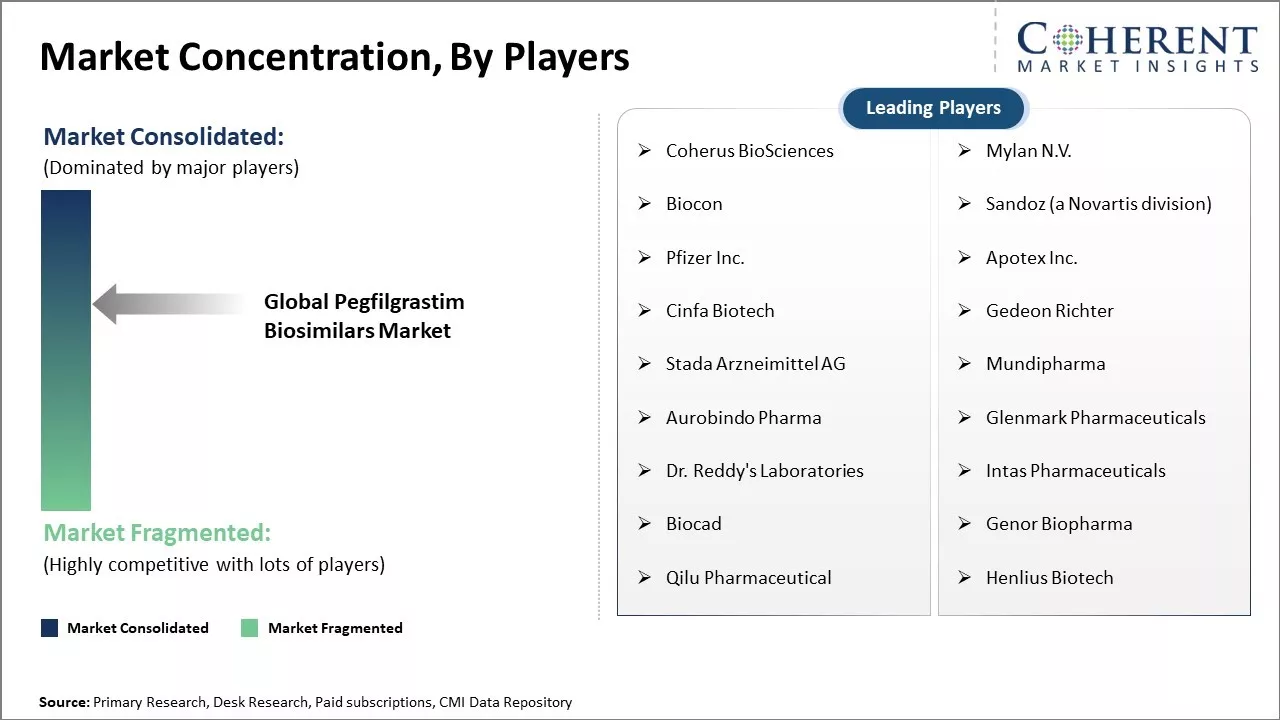
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Patent Expiries of Pegfilgrastim Biosimilars
Several leading brands of pegfilgrastim have lost their patent protection in the recent past, thus, allowing for manufacturing and sale of affordable biosimilar versions. Neulasta, the pioneering pegfilgrastim therapy, lost exclusivity in the U.S. in 2018. This paved way for multiple biosimilar entrants with pegfilgrastim, which are significantly lower priced than the originator brand. Healthcare systems and patients now have access to cheaper biosimilar options without compromising on quality or efficacy. This has boosted overall adoption of pegfilgrastim and volume growth prospects appear promising as physicians and patients increasingly opt for more affordable biosimilars in place of originator versions. Lower prices ensuing from patent expiry of blockbuster brands can facilitate wider global access and uptake of pegfilgrastim biosimilars.
Key Takeaways from Analyst:
Global pegfilgrastim biosimilars market growth is driven by patent expiry of the blockbuster drug- Neulasta and increasing adoption of biosimilars by both physicians and payers. Key regions like Europe and Asia Pacific have laid down regulations to incentivize the uptake of biosimilars, and this drives the market growth.
High entry barriers and stringent regulatory pathway can hamper uptake of pegfilgrastim biosimilars initially. Incumbent competition from current biologics can also pose challenges. Potential anti-substitution laws can jeopardize automatic substitution of biosimilars in some countries. Furthermore, disparities in reimbursement policies can impact pricing and adoption scenarios.
Expanding patient access through cost reductions is set to remain a priority area. Emerging markets like China and India is likely adopt these products faster, buoying global revenues. Moreover, investments in novel drug delivery systems can help differentiate products and appeal to a wider patient base. Collaborations with regional distributors shall also aid market penetration in select territories.
Market Challenges: Regulations and approval processes
Global pegfilgrastim biosimilars market growth can be hampered by various regulatory challenges. Biosimilars are governed by strict regulations related to the approval process and regulations differ from country to country. Meeting the extensive approval criteria set forth by regulatory bodies for biosimilars can be a long and costly process. For instance, in the U.S., the approval pathway for biosimilars is complicated and requires extensive analytical, animal, and clinical studies. Moreover, the regulatory requirements are often ambiguous regarding the extent of similarity evidence required. Such unclear regulations prolong the approval timeline and increase costs for biosimilar companies. Regulations in other regions such as Europe and Japan are also elaborate, which makes the overall approval process time-consuming and capital intensive. The non-uniform regulatory framework across various markets introduces inefficiencies in the approval cycle. These regulatory challenges add to the development and operational costs, which can deter biosimilar manufacturers.
Market Opportunities: Public awareness about benefits of biosimilars
Public awareness about the benefits of biosimilars can offer opportunities for growth of global pegfilgrastim biosimilars market. Biosimilars offer clinically equivalent treatment alternatives to originator biologics at reduced costs, which can significantly expand patient access to important therapies. However, awareness about the safety, efficacy and cost advantages of biosimilars remains relatively low among physicians and patients alike. According to the data from World Health Organization, only 14% of Americans and 10% of Europeans are very or somewhat familiar with biosimilars. Increased education and information dissemination campaigns by regulatory bodies, hospitals, non-profit organizations as well as biosimilar manufacturers have the potential to drive wider acceptance and adoption of biosimilars. For instance, the European Commission has allocated funding for initiatives targeted at improving knowledge about biosimilars among patients and health professionals in member states from 2020-2022. Comprehensive information on the rigorous development process, stringent regulatory pathway and demonstrated similarity to reference products that biosimilars must undergo can help address concerns around their use. This could help providers feel more comfortable in regularly substituting and interchanging therapies with approved pegfilgrastim biosimilars. As familiarity and confidence in biosimilars increases, their potential to deliver significant treatment cost savings will become increasingly attractive to healthcare systems, insurers and providers. The COVID-19 pandemic has placed considerable strain on global healthcare budgets. Expanding the uptake of approved, clinically equivalent and lower cost biosimilars could help free up financial resources that can be reallocated to advance care for other patient populations.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
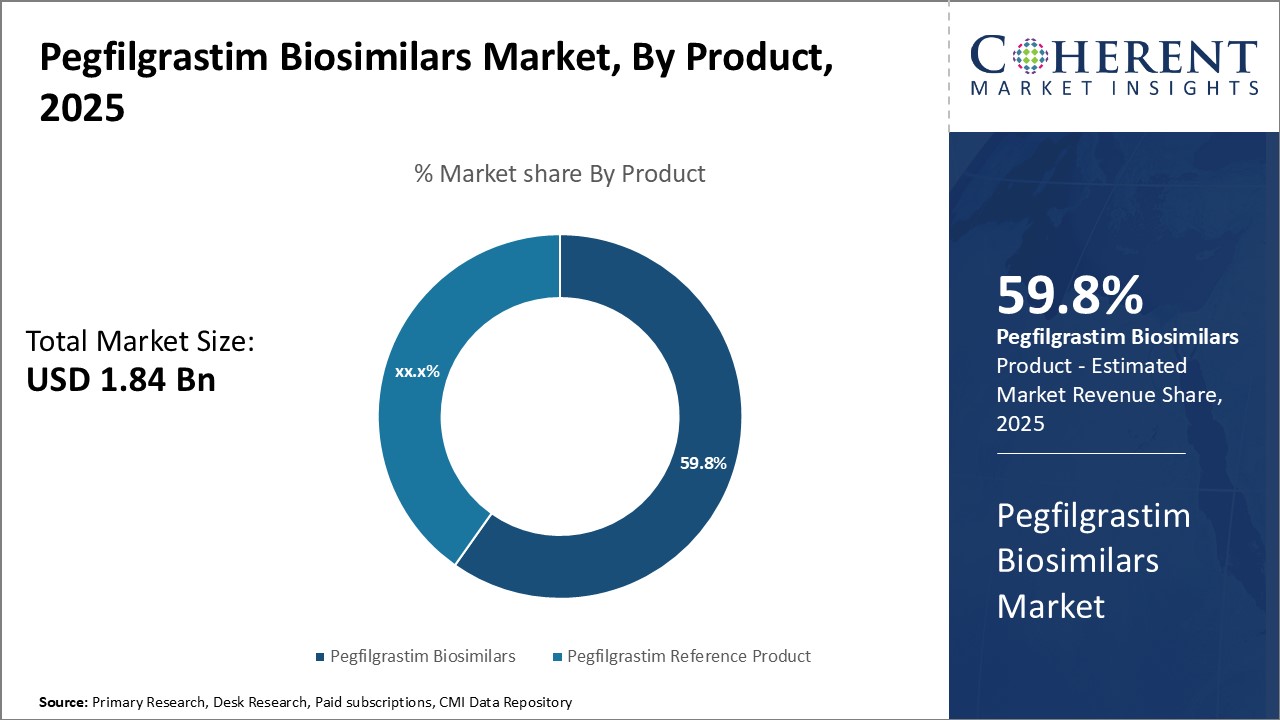
By Product- Rising demand for cost-effective therapies boosts adoption of pegfilgrastim biosimilars
In terms of product, pegfilgrastim biosimilars segment is estimated to contribute the highest market share of 59.8% in 2025, owing to rising demand for cost-effective therapies. Pegfilgrastim Biosimilars provide a more affordable alternative to the reference product for treating chemotherapy-induced neutropenia. Their comparable efficacy and safety profile to the reference product coupled with significant cost savings of up to 30-50% have made them an attractive option for both private and public healthcare systems. This boosts their widespread adoption in developing countries where affordability is a major concern for patients. Recent patent expiry of major pegfilgrastim brands has led to launch of several biosimilar versions by top pharmaceutical companies. This has boosted supply of low-cost biosimilars.
By Application- Oncology dominates due to higher neutropenia risk
In terms of application, oncology segment is estimated to contribute the highest market share of 50.5% in 2025, owing to higher risk of neutropenia faced by cancer patients undergoing chemotherapy. Pegfilgrastim is commonly used to reduce the duration of neutropenia and associated complications like febrile neutropenia in patients receiving myelosuppressive chemotherapy for various cancer types. Neutropenia is a common and sometimes serious side effect of most chemotherapeutic regimens, and this makes G-CSF drugs an essential supportive care for cancer treatment. Rising cancer prevalence globally boosts demand for pegfilgrastim.
By Route of Administration- Subcutaneous route dominates due to ease of self-administration
In terms of route of administration, subcutaneous segment is estimated to contribute the highest market share of 70.5% in 2025, owing to the convenience and ease of self-administration associated with this route. Unlike intravenous pegfilgrastim requiring hospital visits, subcutaneous pegfilgrastim can be self-administered by patients at home after proper training. This reduces the burden on healthcare systems and allows patients to resume normal activities sooner. The subcutaneous route is also associated with fewer side-effects and vascular complications as compared to intravenous delivery. These advantages have boosted preference for the subcutaneous pegfilgrastim especially in out-patient cancer care settings.
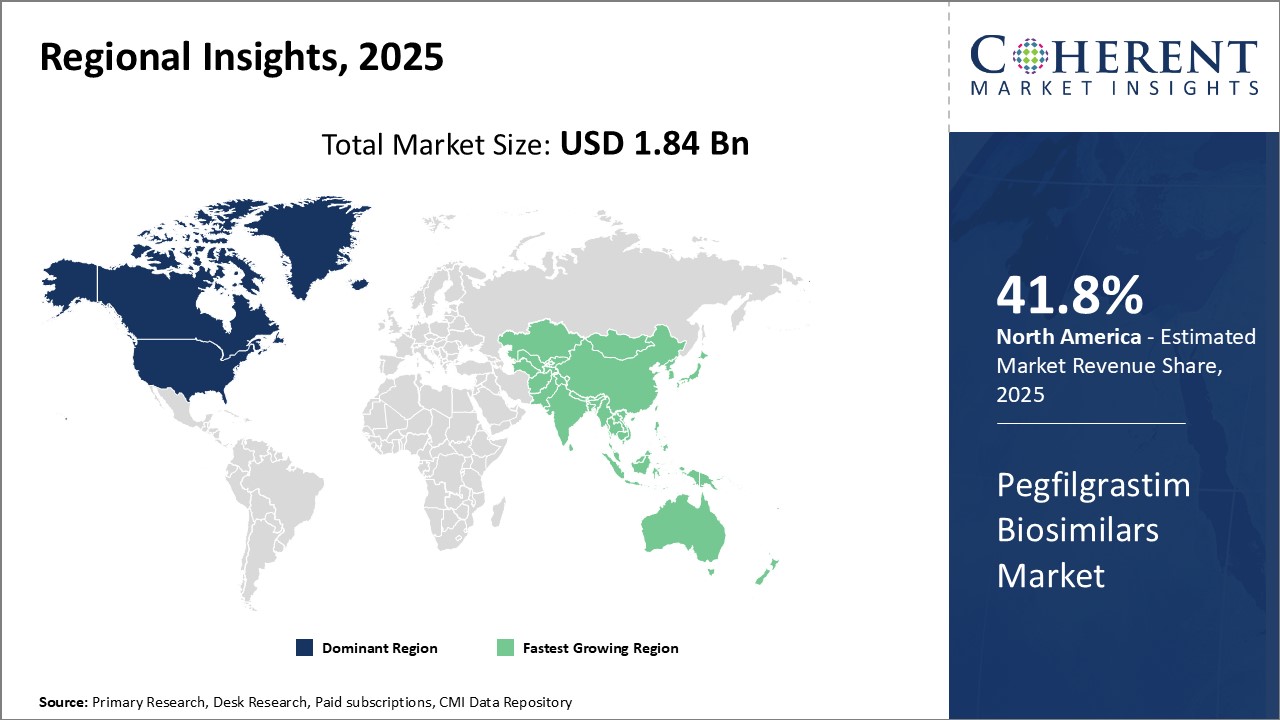
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America dominates the global pegfilgrastim biosimilars market with an estimated market share of 41.8% in 2025. The U.S. market alone accounts for over 40.5% share due to huge demand for biosimilars from healthcare facilities as well as favorable reimbursement policies promoting their usage over reference biologics. Furthermore, presence of large regional pharmaceutical companies with established manufacturing and distribution networks aids product accessibility. While Canada also contributes significantly, future growth may be tempered by pricing pressures from government payers.
Asia Pacific region has emerged as the fastest growing market for pegfilgrastim biosimilars globally over the past few years. Countries such as China, India, South Korea and Japan are witnessing increasing adoption due to growing cancer patient pools as well as massive investments by both domestic and multinational companies to expand local manufacturing capabilities. This ensures reliable and affordable supply to meet domestic treatment needs. The Chinese market exhibit double digit expansion annually with government impetus on developing a strong biosimilars industry under the “Made in China 2025” program.
Market Report Scope
Pegfilgrastim Biosimilars Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.84 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.1% | 2032 Value Projection: | USD 3.39 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Coherus BioSciences, Mylan N.V., Biocon, Sandoz (a Novartis division), Pfizer Inc., Apotex Inc., Cinfa Biotech, Gedeon Richter, Stada Arzneimittel AG, Mundipharma, Aurobindo Pharma, Glenmark Pharmaceuticals, Dr. Reddy's Laboratories, Intas Pharmaceuticals, Biocad, Genor Biopharma, Qilu Pharmaceutical, Henlius Biotech |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pegfilgrastim Biosimilars Industry News
- In December 2023, The U.S. FDA approved Udencya Onbody, an on-body injector for the biosimilar pegfilgrastim-cbqv (Udencya). This device is used to reduce febrile neutropenia risk in cancer patients the day after chemotherapy. Approval was based on extensive data, including pharmacokinetics and bioequivalence studies. Coherus BioSciences plans to launch Udencya Onbody in Q1 2024.
- In May 2023, Amneal Pharmaceuticals launched FYLNETRA (pegfilgrastim-pbbk), a biosimilar to Neulasta in a pre-filled syringe. Developed with Kashiv Biosciences, FYLNETRA is used to treat neutropenia in chemotherapy patients. The product has received an approved J-Code (Q5130) from CMS.
- In June 2020, Pfizer Inc. announced that the USFDA had approved NYVEPRIA (pegfilgrastim-apgf), a biosimilar to Neulasta (pegfilgrastim). NYVEPRIA is indicated to reduce the risk of febrile neutropenia in patients with non-myeloid malignancies undergoing myelosuppressive chemotherapy.
- In December 2023, Coherus BioSciences, Inc. announced that the FDA had approved UDENYCA ONBODY, an on-body injector for UDENYCA (pegfilgrastim-cbqv). This pegfilgrastim biosimilar, administered after chemotherapy, helps reduce the incidence of febrile neutropenia.
*Definition: Global pegfilgrastim biosimilars market consists of pharmaceutical medications that are biosimilar to Neulasta, a long-acting form of the granulocyte colony-stimulating factor (G-CSF) pegfilgrastim. These biosimilar drugs stimulate the bone marrow to produce neutrophils, a type of white blood cell important for fighting bacterial infections. Pegfilgrastim biosimilars are used to treat neutropenia, a low white blood cell count that increases the risk of infections, often seen in patients receiving chemotherapy for cancer treatment.
Market Segmentation
- Product Insights (Revenue, USD Bn, 2020 - 2032)
- Pegfilgrastim Biosimilars
- Pegfilgrastim Reference Product
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Oncology
- Chronic Neutropenia
- Stem Cell Transplantation
- Others
- Route of Administration Insights (Revenue, USD Bn, 2020 - 2032)
- Subcutaneous
- Intravenous
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Coherus BioSciences
- Mylan N.V.
- Biocon
- Sandoz (a Novartis division)
- Pfizer Inc.
- Apotex Inc.
- Cinfa Biotech
- Gedeon Richter
- Stada Arzneimittel AG
- Mundipharma
- Aurobindo Pharma
- Glenmark Pharmaceuticals
- Dr. Reddy's Laboratories
- Intas Pharmaceuticals
- Biocad
- Genor Biopharma
- Qilu Pharmaceutical
- Henlius Biotech
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
