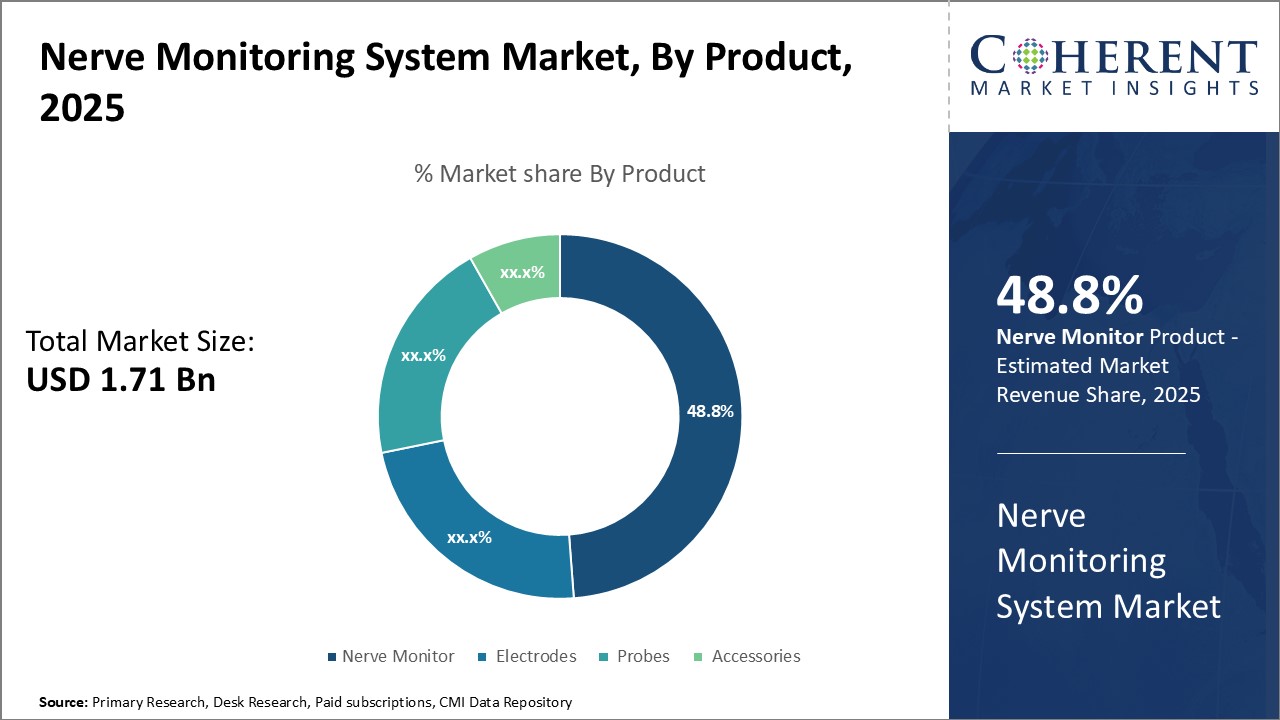
Global nerve monitoring system market is estimated to be valued at USD 1.71 Bn in 2025 and is expected to reach USD 2.55 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.9% from 2025 to 2032.
To learn more about this report, Download Free Sample
Key Takeaways:
- By Product, the nerve monitor segment is projected to lead the global nerve monitoring system market, capturing approximately 48.8% of the market share in 2025.
- By Technology, the electroencephalogram (EEG) segment is expected to dominate the technology landscape, accounting for 39.6% of the market share in 2025.
- By End User, the hospital segment is anticipated to remain the largest end user, securing 54.6% of the market share in 2025.
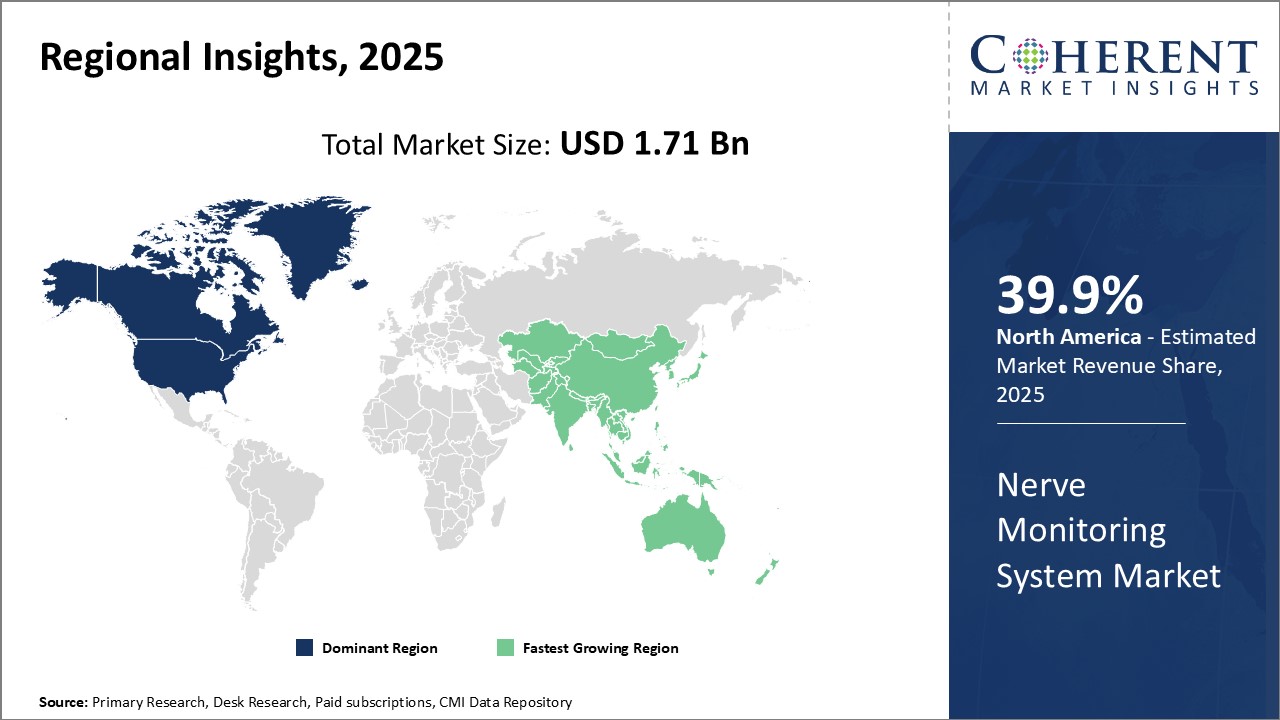
- By Region, North America is forecasted to maintain its dominance in the global nerve monitoring system market, holding 39.9% of the market share in 2025.
Market Overview:
The global nerve monitoring system market is witnessing substantial growth, propelled by the increasing prevalence of neurological disorders, a rise in complex surgical procedures, and growing awareness of intraoperative nerve protection. The market is gaining momentum with continuous advancements in neuromonitoring technologies such as EEG, EMG, and evoked potential systems, which are enhancing surgical precision and patient outcomes.
Current Events and its Impact on the Nerve Monitoring System Market
|
Current Event |
Description and its impact |
|
Rise in Complex Surgical Procedures and Aging Population |
|
|
Advances in Neuromonitoring Technologies |
|
|
Favorable Regulatory and Reimbursement Environment |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pipeline Analysis: Nerve Monitoring System Market
The nerve monitoring system market is witnessing a robust pipeline of innovations aimed at enhancing intraoperative safety and precision. Leading manufacturers such as Medtronic, Nihon Kohden, and Natus Medical are actively developing next-generation systems featuring improved signal clarity, wireless connectivity, and AI-powered predictive analytics.
Several products in the pipeline are focused on minimally invasive procedures, with real-time feedback mechanisms to prevent nerve damage during complex surgeries such as spinal, ENT, and brain operations. Clinical trials are underway for integrated systems that combine nerve monitoring with surgical navigation, enhancing workflow efficiency and outcomes. Moreover, companies are investing in pediatric-specific monitoring devices and disposable electrode technologies to expand clinical applicability.
With strong regulatory momentum and growing clinical demand, the upcoming product pipeline is set to significantly improve surgical safety, reduce post-operative complications, and drive adoption across hospitals and ambulatory surgical centers worldwide. This innovation trajectory is expected to accelerate market growth through 2030.
Patent Landscape: Nerve Monitoring System Market
The nerve monitoring system market exhibits a dynamic patent landscape, driven by ongoing innovation in intraoperative neurophysiological monitoring technologies. Key players such as Medtronic, Nihon Kohden, and NuVasive hold a significant number of patents related to nerve signal detection, electrode placement, real-time data analysis, and integrated monitoring systems.
Recent patent filings focus on AI-enhanced signal interpretation, wireless transmission modules, and minimally invasive sensor designs, highlighting a shift toward smarter and more adaptable surgical tools. Additionally, intellectual property related to disposable and biocompatible electrodes is expanding, aiming to improve patient safety and reduce infection risks. The rise in patents related to multimodal monitoring systems that combine EMG, EEG, and EP technologies reflects the industry’s movement toward comprehensive intraoperative solutions.
Geographically, the U.S., Europe, and Japan lead in patent activity, supported by strong R&D ecosystems. This vibrant patent landscape underscores the sector's innovation potential and competitiveness, fueling future market expansion.
Reimbursement Scenario: Nerve Monitoring System Market
In 2025, the reimbursement landscape for intraoperative nerve monitoring (IONM) systems remains complex, shaped by evolving payer policies and regulatory frameworks. Medicare continues to reimburse IONM services under specific conditions, utilizing CPT codes such as 95940 and G0453.
However, these codes are not reimbursable when billed by the operating surgeon or anesthesiologist, as the services are considered part of the global surgical package. Reimbursement is typically granted when a separate, qualified provider performs the monitoring in designated settings like hospitals or ambulatory surgical centers.
Private insurers, including UnitedHealthcare and Anthem, have established policies that align with Medicare's guidelines, often requiring documentation of medical necessity and adherence to specific place-of-service codes. These policies underscore the importance of compliance with payer-specific requirements to ensure reimbursement.
Prescribers’ Preference: Nerve Monitoring System Market
In 2025, prescribers—particularly neurosurgeons, orthopedic surgeons, and ENT specialists—demonstrate a strong preference for intraoperative nerve monitoring (IONM) systems due to their proven ability to minimize the risk of nerve damage during complex surgeries. These systems are especially favored in high-risk spinal, cranial, and thyroid procedures, where nerve preservation is critical for post-operative quality of life. Surgeons value IONM systems for their real-time feedback, which enhances surgical precision and decision-making during delicate interventions.
There is a noticeable shift toward advanced, user-friendly devices that integrate seamlessly with existing operating room technology. Wireless and multi-modality systems combining EEG, EMG, and evoked potential (EP) monitoring are particularly preferred. Additionally, prescribers lean toward devices supported by strong clinical evidence, favorable training resources, and responsive technical support.
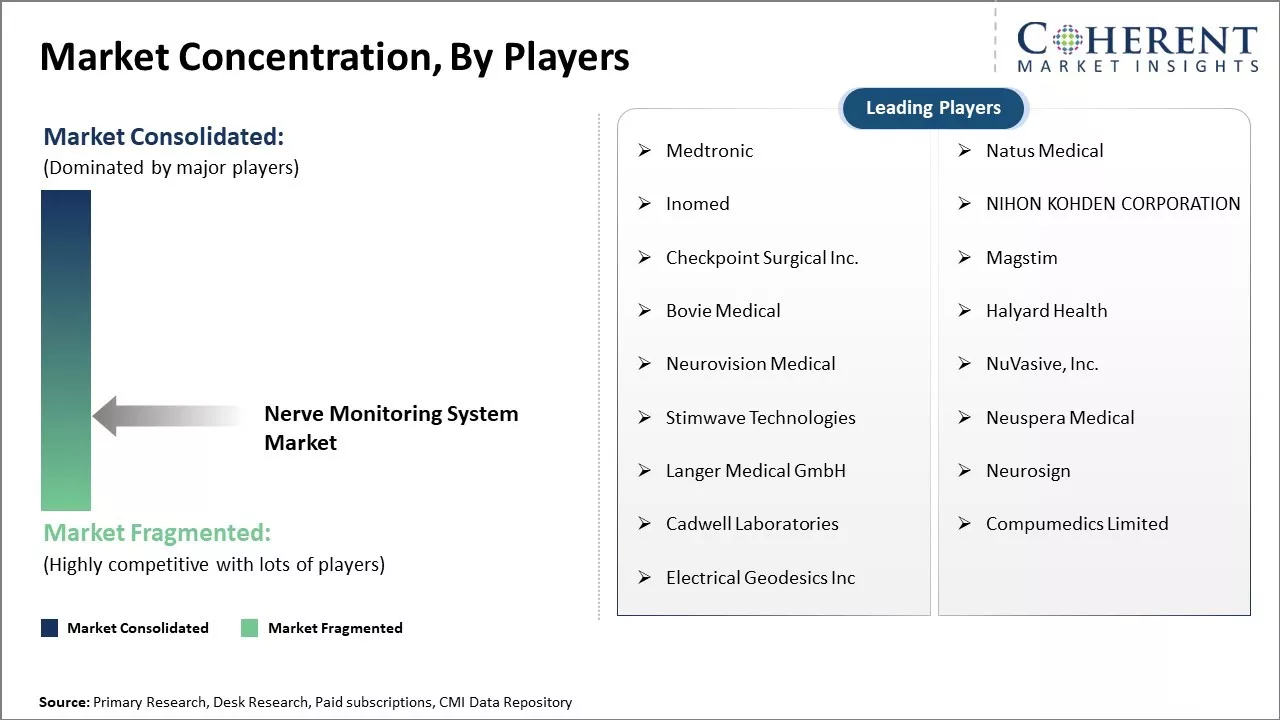
Market Concentration and Competitive Landscape
To learn more about this report, Download Free Sample
Nerve monitoring system market Trends
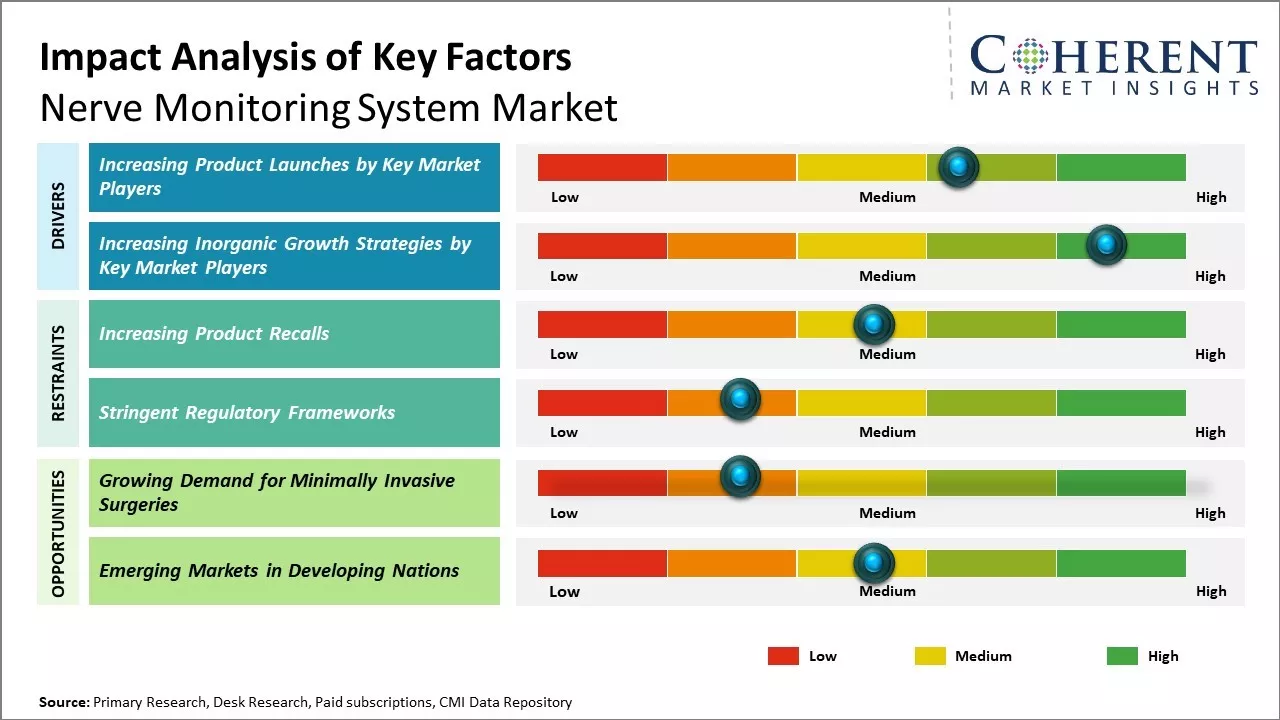
- Increasing Product Launches by Key Market Players
Increasing adoption of organic growth strategies such as product launches by the key market players is expected to drive the market growth over the forecast period. For instance, in May 2021, Neurosign, a medical technology company, launched V4 system for monitoring cranial nerves and spinal nerve roots during surgeries in U.S.
In August 2024, Medtronic recalled a nerve monitoring system after reports indicated that the devices might not emit an alert tone when placed on a nerve, potentially leading wound closure. The company received 70 reports from April 2020 to May 2024 regarding this issue. While the devices remain on the market, Medtronic has issued a software update to address the problem and advised surgeons to rely on alternative methods to prevent nerve damage during procedures.
- Increasing Inorganic Growth Strategies by Key Market Players
Increasing adoption of inorganic growth strategies such as agreements among the key market players is expected to drive the market growth over the forecast period. For instance, in September 2023, Boston Scientific Corporation, a medical device company, announced that it had entered into a definitive agreement with Relievant Medsystems, Inc., a privately held medical technology company, to expand neuromodulation portfolio to provide more treatment options for people living with chronic low back pain.
In May 2025, Auxilium Biotechnologies commenced a pivotal clinical trial for its NeuroSpan Bridge device, aimed at enhancing nerve regeneration. The study plans to enroll 80 patients across the United States to evaluate the device's efficacy and safety in repairing nerve injuries. The NeuroSpan Bridge utilizes a scaffold with microchannels to guide regenerating nerve fibers, potentially offering a novel solution for nerve repair.
Opportunities in the Nerve monitoring system market
- Growing Demand for Minimally Invasive Surgeries
Growing demand for minimally invasive surgeries can offer opportunities for the global nerve monitoring system market growth. There has been a continual shift towards minimally invasive options across different medical specialties over the past decade due to advantages like reduced post-operative pain, shorter hospital stays, and quicker recovery times for patients.
Minimally invasive surgeries are common in many areas like bariatric, hernia repair, and certain orthopedic procedures. However, in complex surgeries that require meticulous handling or dissection of nerves like spine, brain, ENT, or vascular procedures, surgeons are constantly looking for newer techniques and tools to perform such interventions with smaller incisions while ensuring safety.
Nerve Monitoring System Market, By Product Insight
The Nerve Monitor segment is projected to lead the global nerve monitoring system market, accounting for approximately 48.8% of the market share in 2025. This dominance is largely attributed to continuous technological advancements, including the integration of real-time data analysis, improved signal clarity, and compatibility with minimally invasive and robotic surgeries.
These innovations enhance intraoperative precision and patient safety, making nerve monitors increasingly indispensable in high-risk procedures. Furthermore, growing investments in R&D by major players and the expanding adoption of these systems in neurology, orthopedics, and ENT surgeries are reinforcing the segment's market leadership.
Nerve Monitoring System Market, By Technology Insight
The Electroencephalogram (EEG) technology segment is expected to hold the largest share of the nerve monitoring system market in 2025, capturing 39.6%. EEG's ability to provide non-invasive, real-time monitoring of brain activity makes it highly valuable in complex neurosurgical and critical care settings. The increasing volume of high-risk neuro procedures, along with enhanced integration with advanced software platforms, is driving demand for EEG-based systems.
Nerve Monitoring System Market, By End-User Insight
The Hospitals segment is anticipated to dominate the end-user landscape of the nerve monitoring system market in 2025, with a projected market share of 54.6%. This growth is driven by the expansion of private and public healthcare infrastructure and the availability of skilled surgical professionals in hospital settings.
Moreover, favorable reimbursement policies and increasing investments in advanced surgical technologies are promoting the adoption of nerve monitoring systems in hospitals. These systems are becoming standard tools in operating rooms, particularly in neurology, spinal, and orthopedic departments.
Nerve Monitoring System Market: Regional Insight
To learn more about this report, Download Free Sample
North America Nerve Monitoring System Market Trends and Analysis
North America is forecasted to maintain its dominance in the global nerve monitoring system market, holding 39.9% of the market share in 2025. This regional strength stems from robust healthcare infrastructure, a growing prevalence of neurological and orthopedic disorders, and widespread adoption of advanced intraoperative monitoring technologies.
The United States plays a pivotal role, driven by continuous investments in R&D, favorable reimbursement frameworks, and the presence of leading medical device manufacturers. Additionally, increased demand for precision-guided and minimally invasive surgical procedures is propelling the uptake of nerve monitoring systems across the region’s hospitals and surgical centers.
Europe Nerve Monitoring System Market Trends and Analysis
Europe remains a significant region in the nerve monitoring system market, supported by a well-established healthcare network and growing focus on surgical precision and patient safety. Countries such as Germany, the UK, and France are leading adoption, backed by rising neurological disease incidence and national healthcare investments in neuro-monitoring technologies.
Furthermore, active collaboration between academic institutions and medical device companies is enhancing product innovation. Regulatory harmonization and clinical adoption of neurophysiological monitoring standards are expected to drive steady market growth across the continent.
Nerve monitoring system market Dominating Countries:
The United States and Canada dominate the North American nerve monitoring system market, which is projected to account for 39.9% of the global market share in 2025. The U.S. leads the region with its highly advanced surgical infrastructure, high incidence of neurological disorders, and the widespread adoption of intraoperative neurophysiological monitoring (IONM) during complex surgeries.
Leading medical device companies based in the U.S., such as Medtronic and NuVasive, are heavily investing in product innovation and clinical trials to improve surgical outcomes. Canada contributes through its universal healthcare system, rising adoption of precision-guided surgeries, and expanding presence of neuro-monitoring technology in teaching hospitals and tertiary care centers.
Market Report Scope
Nerve Monitoring System Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.71 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.9% | 2032 Value Projection: | USD 2.55 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Natus Medical, Inomed, NIHON KOHDEN CORPORATION, Checkpoint Surgical Inc., Magstim, Bovie Medical, Halyard Health, Neurovision Medical, NuVasive, Inc., Stimwave Technologies, Neuspera Medical, Langer Medical GmbH, Neurosign, Cadwell Laboratories, Compumedics Limited, Electrical Geodesics Inc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Analyst Viewpoint – Nerve Monitoring System Market
Analysts view the nerve monitoring system market as a rapidly expanding sector propelled by continuous technological advancements and the growing demand for safer, precision-guided surgical procedures. The development of multi-modality monitoring systems that combine EEG, EMG, and evoked potential techniques is anticipated to enhance intraoperative accuracy and patient outcomes significantly.
Analysts note that increasing prevalence of neurological disorders and rising volumes of complex surgeries are key growth drivers. Furthermore, supportive reimbursement policies, particularly in North America, and investments in healthcare infrastructure contribute to market expansion. However, challenges such as high device costs, the need for specialized training, and inconsistent adoption in emerging markets could limit growth potential.
Analysts stress the importance of strategic collaborations and innovation in device miniaturization and wireless technologies to boost market penetration. Overall, the outlook remains optimistic, with nerve monitoring systems expected to become an essential tool in surgical practice, improving safety standards and clinical effectiveness globally.
Nerve Monitoring System Market: Key Development
- In March 2025, Medtronic unveiled its next-generation nerve monitoring system, featuring enhanced multi-modality capabilities combining EEG, EMG, and evoked potentials. This innovation improves intraoperative neural mapping accuracy, aiming to reduce surgical risks and improve patient outcomes.
- In April 2025, Natus Medical Incorporated announced a strategic partnership with a leading AI startup to integrate machine learning algorithms into its nerve monitoring devices, enabling real-time predictive analytics during surgeries to optimize neural preservation.
- In February 2025, NeuroVision Technologies received FDA clearance for its wireless, portable nerve monitoring system designed for ambulatory surgical centers, enhancing procedural flexibility and accessibility in outpatient settings.
- In January 2025, Abbott Laboratories expanded its nerve monitoring portfolio by acquiring a neurotechnology firm specializing in advanced electrode design, supporting the development of more precise and minimally invasive neural interfaces.
- In May 2025, a key market player launched an upgraded nerve monitoring software platform featuring cloud connectivity and data analytics, facilitating better clinical decision-making and post-operative patient management.
Key Players Insights
- Medtronic
- Natus Medical
- Inomed
- NIHON KOHDEN CORPORATION
- Checkpoint Surgical Inc.
- Magstim
- Bovie Medical
- Halyard Health
- Neurovision Medical
- NuVasive, Inc.
- Stimwave Technologies
- Neuspera Medical
- Langer Medical GmbH
- Neurosign
- Cadwell Laboratories
- Compumedics Limited
- Electrical Geodesics Inc
Sources
The Stakeholders Consulted:
• Neurosurgeons and surgical teams specializing in neurological procedures
• Medical device manufacturers and suppliers of nerve monitoring systems
• Biomedical engineers and clinical technicians
• Hospital procurement and healthcare administration professionals
• Regulatory agencies overseeing medical devices and patient safety
• Research institutions focused on neurotechnology and intraoperative monitoring
• Healthcare policy makers and reimbursement experts
Databases Accessed:
• U.S. Food and Drug Administration (FDA) – Medical Device Databases
• National Institutes of Health (NIH) – Clinical Trials and Medical Research Data
• World Health Organization (WHO) – Global Health Statistics and Device Usage
• European Medicines Agency (EMA) – Medical Device Approvals and Safety Data
Magazines & Trade Publications:
• Neurology Today
• Medical Device and Diagnostic Industry (MD+DI)
• Journal of Clinical Monitoring and Computing
• Healthcare Technology Management Magazine
• OR Manager – Operating Room News and Equipment Reviews
Scientific and Industry Journals:
• Journal of Neurosurgery
• Neuromonitoring
• IEEE Transactions on Biomedical Engineering
• Clinical Neurophysiology
• Surgical Technology International
Newspapers & Media Outlets:
• The New York Times – Health & Science Section
• Reuters Health – Medical Technology Updates
• The Guardian – Healthcare Innovation
• Bloomberg Healthcare – Medical Devices and Technologies
• The Times of India – Healthcare Developments
Associations and Regulatory Bodies:
• U.S. Food and Drug Administration (FDA)
• European Commission – Medical Device Regulation (MDR)
• International Neuromonitoring Society (INMS)
• Association for the Advancement of Medical Instrumentation (AAMI)
• National Institute for Health and Care Excellence (NICE)
Public Domain Sources:
• Centers for Medicare & Medicaid Services (CMS) – Reimbursement Data
• World Health Organization (WHO) – Medical Device Reports and Guidelines
• National Health Service (NHS) – Device Adoption and Clinical Guidelines
• Global Medical Device Nomenclature (GMDN) – Device Classification
Proprietary Research Elements:
• In-depth Expert Interviews with Neurosurgeons, Biomedical Engineers, and Healthcare Administrators
• CMI Analytics Platform – Market Trends and Forecast Modeling
• Comprehensive Market Survey Data covering Healthcare Providers and End-Users
• Case Studies and Clinical Outcome Analyses related to Nerve Monitoring System Adoption
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients