Nephrology Drugs Market Size and Trends
Global nephrology drugs market is estimated to be valued at USD 18.37 Bn in 2025 and is expected to reach USD 29.14 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032. Growing prevalence of kidney diseases such as chronic kidney disease, diabetes mellitus, and polycystic kidney disease globally can drive the nephrology drugs market growth during the forecast period.
Discover market dynamics shaping the industry: Download Free Sample
The market is witnessing growth due to increasing investment by leading manufacturers for the development of novel drugs to treat kidney disorders. Furthermore, rising awareness about kidney diseases and availability of various treatment options can offer opportunities for the market players. However, stringent regulatory frameworks for the approval of nephrology drugs can hamper the market growth.
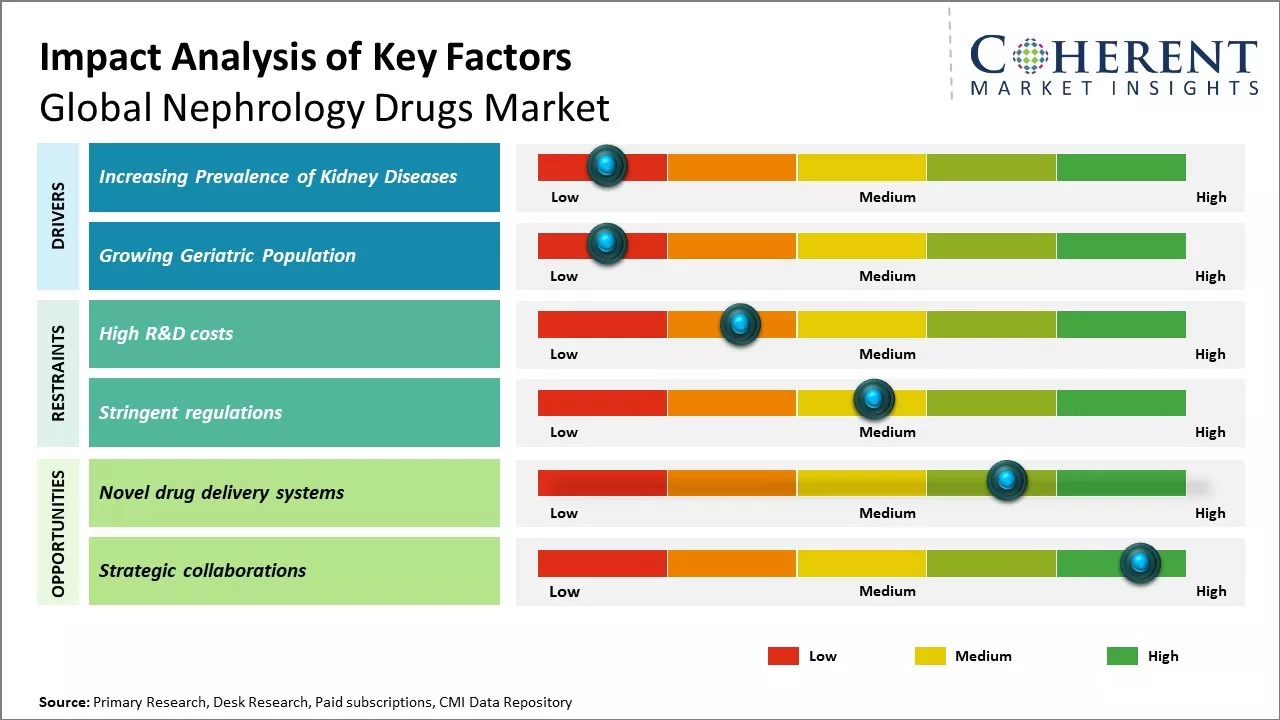
Market Driver – Increasing Prevalence of Kidney Diseases
Global nephrology drugs market is witnessing significant growth due to increasing incidence and prevalence of various kidney diseases across the world. Kidney diseases have become a major global health problem with an increasing trend observed in both developed and developing countries. According to the data published by WHO, chronic kidney disease affected approximately 10% of the world's population in 2020. The major risk factors attributing to kidney diseases include rising cases of obesity, prevalence of diabetes and hypertension, aging population, and growing environmental pollutants. These factors have triggered various forms of chronic and end-stage kidney diseases, resulting in increased need for dialysis treatment and kidney transplant procedures. Rising patient pool suffering from conditions like chronic kidney failure, glomerulonephritis, polycystic kidney disease, and others has boosted demand for varied nephrology drugs used for both acute and chronic management of symptoms. Pharmaceutical companies have channeled more resources towards development of novel drugs for effective treatment of kidney disorders. For instance, according to an article published by International Society of Nephrology (ISN), there has been rise in prevalence of kidney diseases with more than 850 million people worldwide affected by the disease. Chronic Kidney Disease (CKD) affects approximately 10.4% of men and 11.8% of women globally, while acute kidney injury (AKI) annually impacts 13.3 million people, a figure that underscores the acute nature of the problem. Rising prevalence of kidney diseases not only poses a substantial burden on healthcare systems but also underscores the urgent need for enhanced public awareness, early detection strategies, and accessible treatment options to mitigate the escalating impact of this silent epidemic.
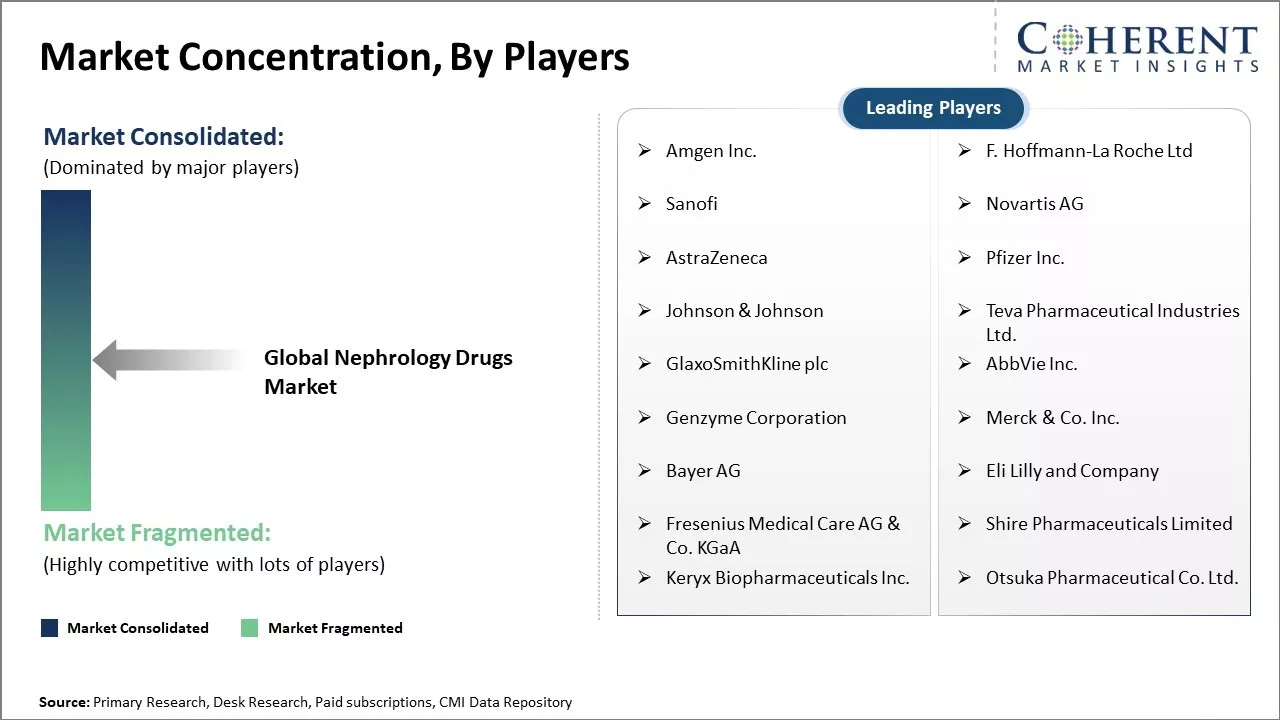
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Growing Geriatric Population
Rising geriatric population worldwide, who are more susceptible to developing kidney diseases, can drive the market growth. Older adults often have coexisting conditions such as hypertension, cardiovascular diseases, and diabetes which further elevate the risk. Moreover, aging physiology makes the kidneys less efficient over time. According to the data from United Nations, there were over 727 million people aged 65 years and above globally in 2020, which accounted for nearly 9% of the world's population. This geriatric demographic is expected to double to 1.5 billion by 2050. Aged individuals require more vigilant nephrology care and long term pharmacotherapy using multiple drugs. With rising life expectancy, there will be increase in consumption of nephrology medications by elderly population. Expanding aging demographics coupled with associated complex medical conditions can drive the market growth.
Key Takeaways from Analyst:
Global nephrology drugs market growth is driven by rising prevalence of kidney diseases worldwide. Rising geriatric population who are more susceptible to chronic kidney diseases can boost demand for nephrology drugs. Growing awareness about kidney ailments and availability of various treatment options can drive the market growth. However, stringent regulations for drug approval and high costs associated with R&D can hamper the market growth.
North America currently dominates the nephrology drugs due to growing incidences of kidney disorders, rising healthcare spending, presence of advanced medical facilities and favorable reimbursement policies in the region. Asia Pacific is likely to witness the fastest growth, owing to expanding patient pool, increasing healthcare spending, growing medical tourism and rising disposable income.
Emerging economies provides lucrative opportunities for nephrology drugs manufacturers due to growing unmet needs. Companies need to strengthen their distribution channels to efficiently cater to growing patient base. Further collaborations and acquisitions could aid players expand their product portfolios and geographic footprint.
Market Challenges: High R&D costs
High research and development costs involved in developing new nephrology drugs can hamper the global nephrology drugs market growth. Developing new drugs requires extensive research, testing, clinical trials and regulatory approvals which involves significant financial investments over long periods of time. It takes over 10-15 years for a new drug to be developed and brought to the market from the point of discovery. According to the data published by World Health Organization, the . total costs associated with developing a new drug from scratch can range anywhere between US$ 250-350 million. A lot of these costs are spent during the clinical trial phases where human testing of potential drug candidates are conducted to evaluate safety and efficacy. Only a small fraction of drug candidates make it past each phase of clinical trials and gain regulatory approvals. Most drug candidates fail during the costly clinical trial phases itself, which results in huge financial losses for pharmaceutical companies. This high risk of failure associated with the R&D process discourages investments in development of new nephrology drugs intended for rare diseases and conditions affecting smaller patient segments.
Market Opportunities: Novel drug delivery systems
Novel drug delivery systems can offer opportunity for global nephrology drugs market growth. New technologies that allow for targeted, controlled release of medications over extended periods can help improve treatment outcomes for chronic kidney diseases. Many kidney conditions require lifelong management through frequent drug interventions. However, traditionally available administration methods like oral pills or injections only achieve brief periods of therapeutic concentrations in the body. These also have issues with patient compliance since strict dosing schedules must be followed. Novel delivery mechanisms which embed drugs inside nanocarriers, microspheres or implantable depots could revolutionize care. Medications encapsulated in such innovative formulations get automatically transported to organs or tissues of interest. These then release pharmaceutical substances in a pre-programmed, sustained manner over weeks or months from a single administration.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
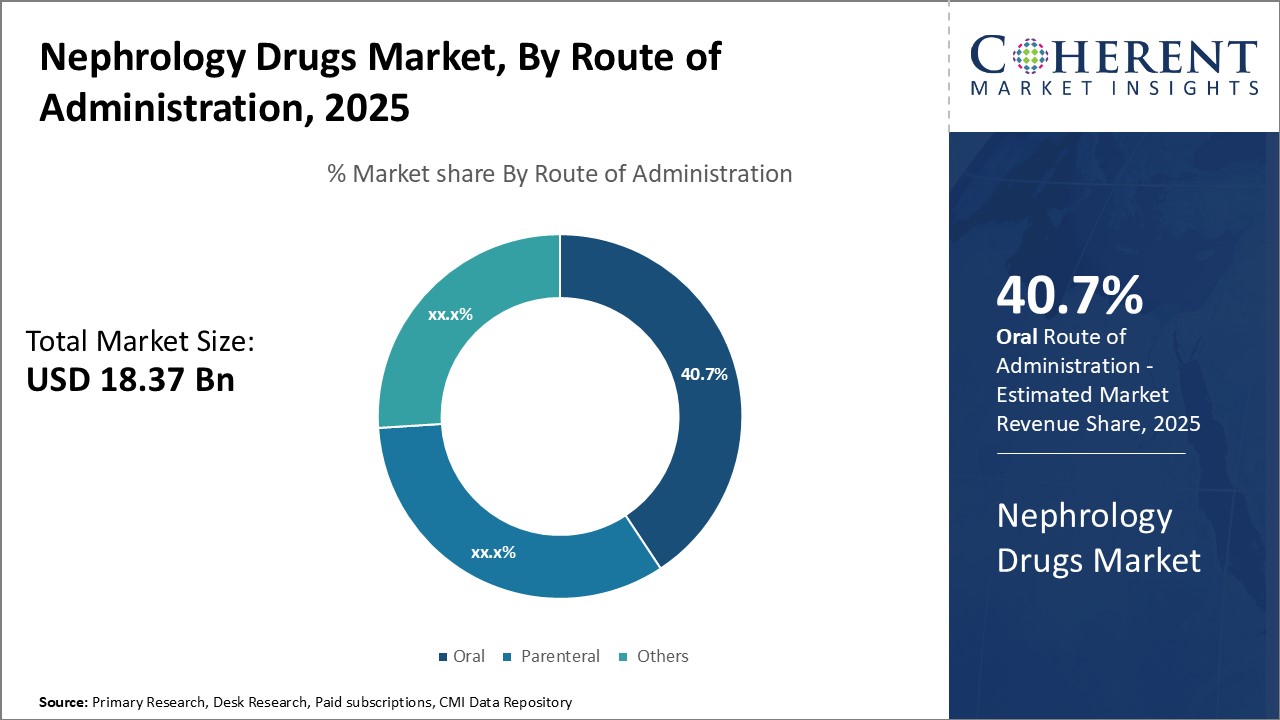
Insights, By Route of Administration: Convenience and cost-effectiveness can drive oral segment growth
In terms of route of administration, oral segment is estimated to contribute the highest market share of 40.7% in 2025, owing to the convenience and cost-effectiveness it offers to patients and caregivers. Oral drugs are easier to administer as compared to parenteral drugs which require administration through injection or intravenous methods. This reduces the burden on patients who can self-administer oral medications at home. It also removes the need for frequent hospital or clinic visits only for drug administration. The oral route avoids pain and discomfort associated with injections as well as risks of complications from parenteral administration such as infections. Moreover, oral drugs have lower distribution and storage costs as compared to parenteral drugs since these do not require strict temperature controls or dedicated medical professionals for administration. This makes oral drugs more affordable for patients and the healthcare system. The non-invasive nature and economic advantages of oral drugs have made them the preferred choice among patients and healthcare providers in the management of chronic conditions like nephrological disorders.
Insights, By Drug Class - ACE Inhibitors dominate due to wide indications and clinical efficacy
In terms of drug class, ACE Inhibitors segment is estimated to contribute the highest market share of 25.71% in 2025, owing to their wide range of indications and proven clinical efficacy. ACE Inhibitors are recommended as the first-line treatment for numerous chronic kidney disease conditions like diabetic nephropathy, hypertension with kidney disease, and chronic kidney disease regardless of cause. These effectively lower blood pressure and slow the progression of kidney damage. ACE Inhibitors are also used to treat heart failure and reduce cardiovascular risks. Their ability to target multiple complications of kidney diseases through a single medication makes them extremely valuable. Decades of clinical research have established ACE Inhibitors as safe and effective therapies. This widespread use across indications and strong evidence-base have made ACE inhibitors the mainstay of pharmacological management for nephrological disorders.
Insights, By Distribution Channel - Increased access and compliance drive hospital pharmacy segment
In terms of distribution channel, hospital pharmacy segment is estimated to contribute the highest market share of 40.62% in 2025, owing to increased access to care and ensured treatment compliance it provides. In many countries, hospital pharmacies serve as the primary point of contact for patients with chronic kidney conditions to receive specialized nephrological drugs as hospitals employ renal specialists, conduct advanced diagnostic tests, and have infrastructure for long-term care. Utilizing hospital pharmacies also helps ensure patients receive proper counseling and education about medication use from clinical pharmacists. It aids in monitoring drug adherence and managing complications under medical guidance. For patients on complex drug regimens, the controlled environment of hospitals increases therapeutic compliance Hospital pharmacies play a critical role in expanding access to important nephrology medications and optimizing their real-world effectiveness through a multidisciplinary team approach.
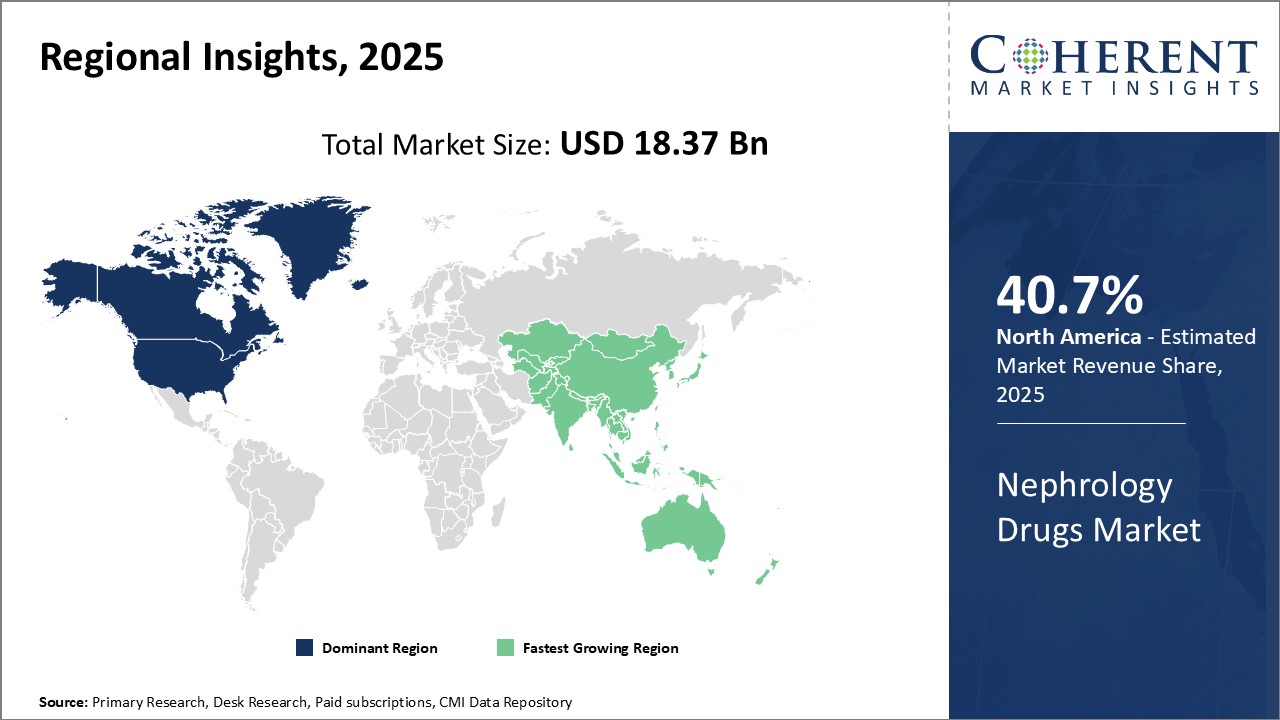
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America dominates the nephrology drugs market with an estimated market share of 40.7% in 2025. Major pharmaceutical companies from the U.S. and Canada have a strong presence in this region with widespread R&D facilities and production bases. With high healthcare spending and advanced medical infrastructure, patients in the U.S. and Canada have easy access to innovative and advanced treatment options. In terms of pricing and availability of nephrology drugs, North America leads the world with little to no price control imposed by governments. This ensures that pharmaceutical companies can recoup heavy R&D investments through established pricing mechanisms in the region.
Asia Pacific market, led by China, India and Japan, is witnessing the fastest growth and emerging as the next major hub for nephrology drugs. Growing burden of kidney diseases mainly due to rising geriatric population, increasing healthcare expenditure, growing medical tourism and rising focus of international drug makers on these developing markets can also drive the market growth. China and India are heavily reliant on imports to meet their massive requirements as domestic production cannot keep up the pace. However, countries like China, India and South Korea are bolstering their domestic pharmaceutical industry through initiatives like 'Make in China' and 'Make in India' to reduce import dependence over the long term.
Market Report Scope
Nephrology Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 18.37 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 29.14 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Amgen Inc., F. Hoffmann-La Roche Ltd, Sanofi, Novartis AG, AstraZeneca, Pfizer Inc., Johnson & Johnson, Teva Pharmaceutical Industries Ltd., GlaxoSmithKline plc, AbbVie Inc., Genzyme Corporation, Merck & Co. Inc., Bayer AG, Eli Lilly and Company, Fresenius Medical Care AG & Co. KGaA, Shire Pharmaceuticals Limited, Keryx Biopharmaceuticals Inc., Otsuka Pharmaceutical Co. Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Nephrology Drugs Industry News
- On January 17, 2024, Sun Pharma, a pharmaceutical company, announced a partnership with Bayer AG, a Germany-based multinational pharmaceutical and biotechnology company, to market a drug designed for treating chronic kidney disease and other conditions. As per the agreement, Bayer AG has provided Sun Pharma with non-exclusive rights to distribute and market a second Finerenone product named Lyvelsa.
- Everest Medicines, a biopharmaceutical firm specializing in discovering, clinically developing, manufacturing, and commercializing innovative medicines and vaccines, announced the successful launch of NEFECO in China. This launch signifies a significant advancement in patient care for IgA nephropathy (IgAN) in the region.
- The Central Drugs Standard Control Organisation (CDSCO) had granted approval for Jardiance (empagliflozin) 10 mg tablets. This approval allows for the reduction of the risk of sustained decline in individuals with end-stage kidney disease, cardiovascular death, and hospitalization in adults diagnosed with chronic kidney disease (CKD) who are at risk of disease progression.
- Novartis, a innovative medicines company, presented results from the Phase III APPLAUSE-IgAN study of Fabhalta (iptacopan) at the World Congress of Nephrology in Buenos Aires, Argentina. Fabhalta, an investigational Factor B inhibitor for IgA nephropathy (IgAN), showed promising outcomes. Patients treated with Fabhalta achieved a significant 38.3% reduction in proteinuria (as measured by 24-hour urine protein to creatinine ratio [UPCR]) at 9 months compared to those receiving placebo alongside standard supportive care (p<0.0001).
*Definition: Global nephrology drugs market consists of pharmaceutical drugs used for the treatment of kidney diseases and kidney related disorders. This market includes drugs for various kidney diseases such as chronic kidney disease, renal disease, kidney stones, urinary tract infections, and others. The nephrology drugs market has been growing steadily due to the rising prevalence of chronic kidney diseases worldwide and an aging global population that is more prone to developing kidney problems. Key players in this market are focusing on developing novel drug formulations and treatment options to cater to the growing patient pool affected by nephrological conditions.
Market Segmentation
- Route of Administration Insights (Revenue, USD Bn, 2020 - 2032)
- Oral
- Parenteral
- Others
- Drug Class Insights (Revenue, USD Bn, 2020 - 2032)
- ACE Inhibitors
- Angiotensin Receptor Blockers (ARBs)
- B-Blockers
- Calcium Channel Blockers
- Loop Diuretics
- Erythropoiesis-Stimulating Agents (ESAs)
- Phosphate Binders
- Others
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Amgen Inc.
- F. Hoffmann-La Roche Ltd
- Sanofi
- Novartis AG
- AstraZeneca
- Pfizer Inc.
- Johnson & Johnson
- Teva Pharmaceutical Industries Ltd.
- GlaxoSmithKline plc
- AbbVie Inc.
- Genzyme Corporation
- Merck & Co. Inc.
- Bayer AG
- Eli Lilly and Company
- Fresenius Medical Care AG & Co. KGaA
- Shire Pharmaceuticals Limited
- Keryx Biopharmaceuticals Inc.
- Otsuka Pharmaceutical Co. Ltd.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
