Monkeypox (Mpox) Diagnosis Market Size and Trends
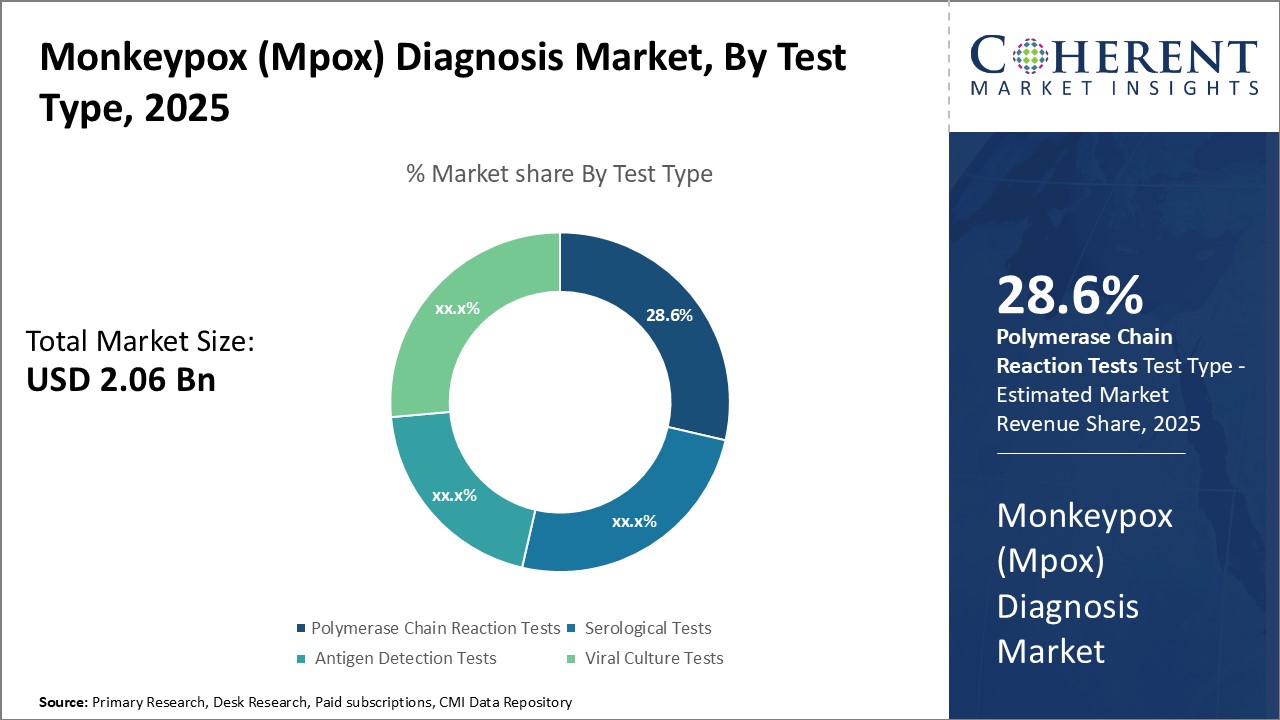
Global monkeypox (Mpox) diagnosis market is estimated to be valued at USD 2.06 Bn in 2025 and is expected to reach USD 2.78 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 4.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
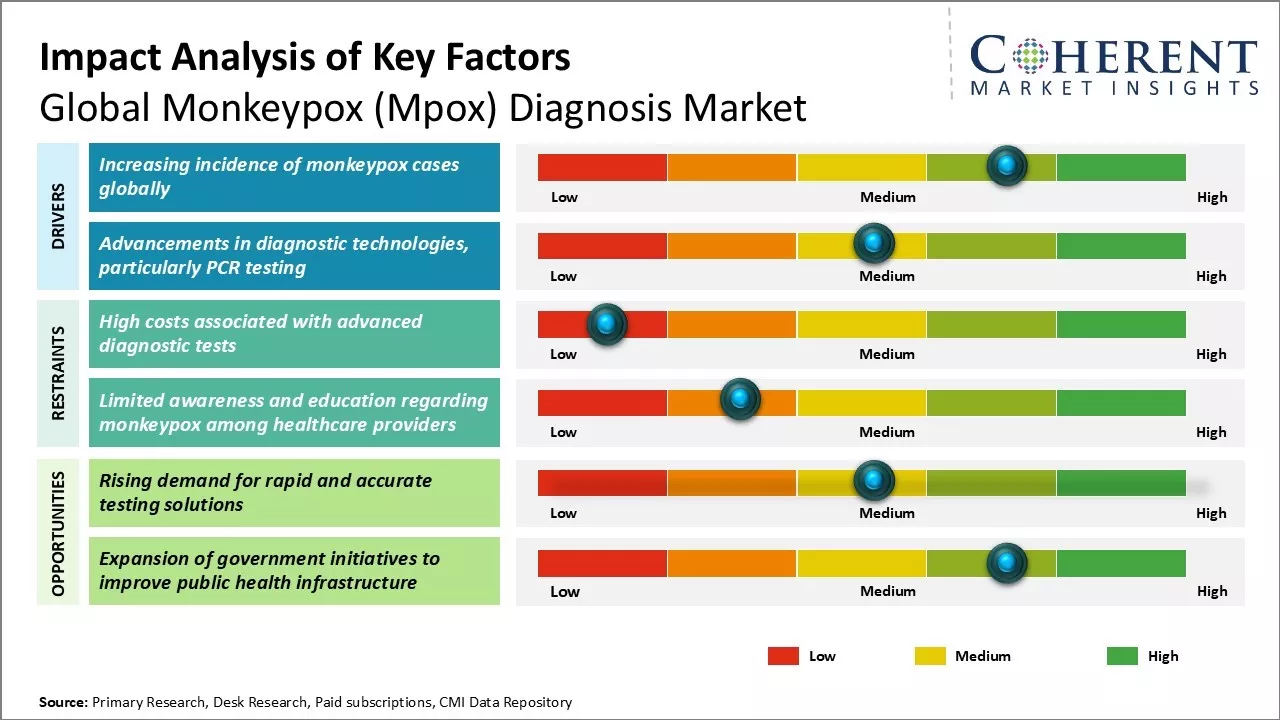
With rising monkeypox outbreak globally, the need for rapid, sensitive, and accurate monkeypox diagnosis has increased tremendously. Various factors such as increasing R&D investments by key players for the development of novel diagnostic kits, growing public awareness about mpox infection, and availability of reimbursements for mpox diagnostic tests are expected to drive the market growth. However, lack of awareness in developing nations, high cost of diagnostic tests and delay in test results associated with conventional diagnosis may hamper the market growth.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Test Type - Advancement in molecular diagnostic techniques drives polymerase chain reaction tests
Polymerase chain reaction (PCR) tests segment lead the market with share of 28.6% in 2025, due to advancements in molecular diagnostics. They provide accurate detection of the monkeypox virus by replicating its genetic material. Real-time quantitative polymerase chain reaction assays enable rapid viral load measurement, while isothermal amplification methods offer polymerase chain reaction benefits without thermal cycling. Multiplex polymerase chain reaction kits allow for simultaneous detection of monkeypox and other orthopox viruses. Widespread use of automated polymerase chain reaction platforms accelerates diagnostics, with ongoing research in syndromic panels expanding their applications.
Insights, By Mode - Centralized healthcare infrastructure favors laboratory testing
Laboratory testing segment holds the largest market share with 52.62% in 2025, due to established centralized healthcare infrastructure. Suspected monkeypox cases are typically screened and tested in clinical or public health labs with Biosafety Level-2 containment. These labs have the necessary equipment, trained personnel, and standardized procedures for orthopox virus diagnostics. High testing volumes support automation and multiplexing. Specimens from remote areas or airports can be transported to reference labs, while mobile testing vans expand access. However, regulatory challenges limit point-of-care testing.
Insights, By Component - Sophisticated hardware enables advanced diagnostics
Hardware segment leads the market share with 54.6% in 2025, due to continuous innovation in diagnostic technologies. Advanced real-time polymerase chain reaction machines, integrated centrifuges, and viral load quantification platforms enhance diagnostic speed and accuracy. Digital imaging microscopes and robotic liquid handling stations improve lab efficiency. Portable devices with multiple assays support field operations, while cloud-based hardware aids telehealth implementation. Favorable regulations encourage new product development, driving the global monkeypox (mpox) diagnosis market through technological advancements in diagnostic hardware.
Regional Insights
Need a Different Region or Segment? Download Free Sample
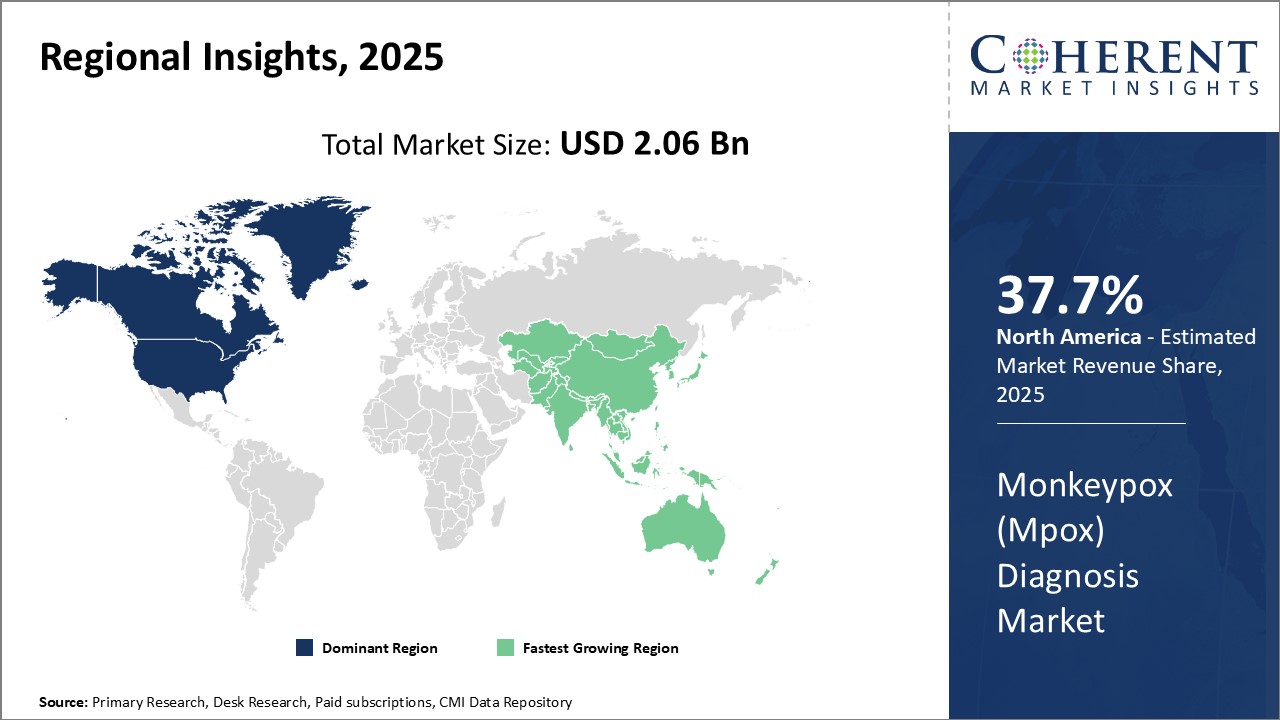
Dominating Region: North America
North America dominates the monkeypox (Mpox) diagnosis market with share of 37.7% in 2025, which can be attributed to strong local healthcare infrastructure and government support for disease control and prevention. Additionally, the region is home to many leading medical technology companies that are actively engaging in the development and commercialization of diagnostic solutions for emerging health threats.
Fastest-Growing Region: Asia Pacific
Asia Pacific region exhibits the fastest growth in this market. Rapid economic development and rising healthcare spending have strengthened regional capabilities to address public health priorities. At the same time, population growth and dense urbanization in many Asian countries increase the risks of infectious disease outbreaks, driving the need for robust diagnostic preparedness.
Monkeypox (Mpox) Diagnosis Market Outlook for Key Countries
Diagnostic Leadership in the U.S.
The U.S. leads in monkeypox diagnostic innovation, bolstered by an advanced healthcare infrastructure and swift test development initiatives. In September 2023, Hologic, Inc. received Emergency Use Authorization (EUA) for its monkeypox test, significantly enhancing diagnostic response capacities across the country. The U.S. Centers for Disease Control and Prevention (CDC) has actively supported the implementation of rapid testing systems, ensuring robust diagnostic solutions are available nationwide, thereby setting a benchmark for global disease surveillance and response.
Strong Public Health Framework in Canada
Canada’s proactive public health approach includes enhanced surveillance and diagnostics for managing monkeypox outbreaks. In July 2023, the Public Health Agency of Canada (PHAC) expanded testing capabilities to address the rise in cases, underscoring the nation’s commitment to effective outbreak management. Through collaboration with provincial health agencies, Canada has strengthened its monitoring systems and implemented public health campaigns, aiming to detect and contain cases swiftly to protect vulnerable communities and prevent further spread.
Strengthening Disease Surveillance in Japan
Japan has adopted a cautious yet robust approach to monitoring zoonotic diseases, including monkeypox, focusing on preparedness and early response. In August 2023, Japan’s Ministry of Health announced enhanced disease surveillance and response strategies to detect zoonotic infections promptly. These initiatives include improved laboratory capacity, integrated diagnostic systems, and public health education programs. Japan’s proactive stance underscores its commitment to preventing potential outbreaks, safeguarding public health, and fostering a resilient healthcare framework for emerging infectious diseases.
Expanding Diagnostic Infrastructure in China
China's commitment to expanding diagnostic infrastructure is a major driver of market growth, fueled by government initiatives and high healthcare demand. Large-scale investments in modernizing healthcare facilities and integrating advanced diagnostic technologies are enhancing the accessibility and quality of medical services across urban and rural areas. Additionally, China's focus on AI-driven diagnostics and digital health solutions is accelerating early disease detection and treatment efficiency.
Focus on Awareness and Test Development in India
India's emphasis on raising awareness about healthcare and investing in test development is a significant driver of market growth. Government campaigns and healthcare initiatives, such as Ayushman Bharat, are improving public understanding of disease prevention and the importance of early diagnosis. Simultaneously, India is focusing on the development of affordable, accurate diagnostic tests, supported by both local innovation and collaborations with global entities. This combination of awareness-building and test advancements is driving greater adoption of diagnostic solutions, fueling market expansion across the country.
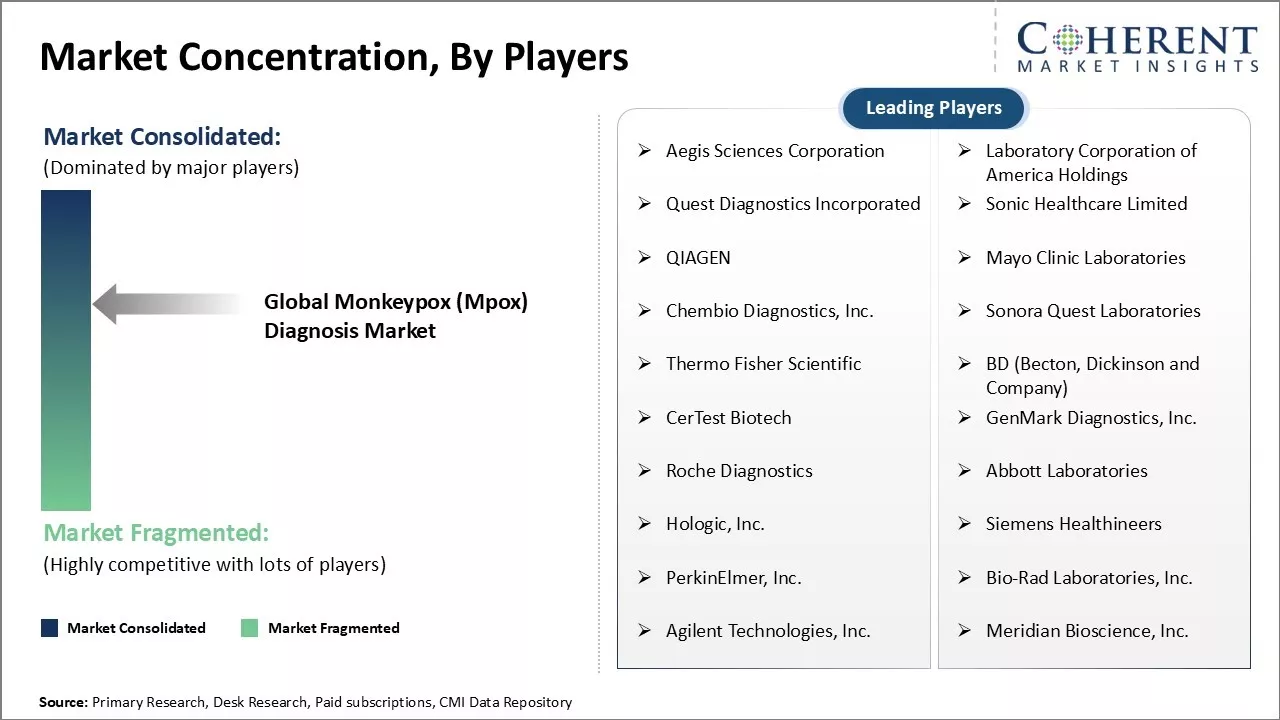
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Global Monkeypox (Mpox) Diagnosis Market Players
- Established players have invested heavily in R&D to innovate industry-leading diagnostic solutions. Major companies like Abbott Laboratories and QIAGEN allocate over 15% of annual revenues to develop advanced products like polymerase chain reaction-based tests with higher accuracy and rapid turnaround times.
- Mid-level players stay competitive through cost-effective strategies. Companies like Siemens Healthineers focus on affordability without compromising quality. Their portfolio includes affordable immunoassays and antigen tests priced 20-30% lower than market leaders.
- Smaller participants have found niche success through technological differentiation. Abbott excels in portable diagnostics using microfluidics. Their handheld molecular diagnostic device provides lab-quality Mpox detection from non-clinical settings within an hour.
Emerging Startups in the Global Monkeypox (Mpox) Diagnosis Market
Several startups are bringing innovative technologies. Erba Transasia Diagnostics applies AI to digitally analyze patient samples, reducing diagnostic time from days to minutes. Qiagen leverages pathogen genomics, with databases to track virus evolution, aiding vaccine and therapeutic development.
Sustainability-focused startups are also emerging. Chembio Diagnostics, Inc. produces biodegradable test kits from renewable materials like bagasse and cornstarch. Their production process utilizes solar power, lowering carbon footprint by 60%.
Startups effectively address niche needs. While larger firms focus on general public health, sonic healthcare aids research through high-throughput mPox testing solutions. They partnered with universities developing animal models, expediting non-human primate studies.
Collaborations are also key for startups. Hologic Inc. joined a government incubator program, gaining expertise and pilot funding and the opportunity to trial their microarray-based test.
Key Takeaways from Analyst
- The recent monkeypox outbreak has boosted the demand for reliable diagnostics, with North America leading due to high case numbers. polymerase chain reaction tests, the primary diagnostic method, are offered by companies like Roche, Abbott, and BioMerieux, but require specialized labs and processing time, creating a need for faster solutions. Point-of-care antigen tests are gaining interest but face regulatory and funding challenges. Additionally, symptom overlap with conditions like chickenpox and undetected rashes complicates accurate diagnosis, highlighting the potential for combined DNA and antibody detection.
Market Report Scope
Monkeypox (Mpox) Diagnosis Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.06 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.4% | 2032 Value Projection: | USD 2.78 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Aegis Sciences Corporation, Laboratory Corporation of America Holdings, Quest Diagnostics Incorporated, Sonic Healthcare Limited, QIAGEN, Mayo Clinic Laboratories, Chembio Diagnostics, Inc., Sonora Quest Laboratories, Thermo Fisher Scientific, BD (Becton, Dickinson and Company), CerTest Biotech, GenMark Diagnostics, Inc., Roche Diagnostics, Abbott Laboratories, Hologic, Inc., Siemens Healthineers, PerkinElmer, Inc., Bio-Rad Laboratories, Inc., Agilent Technologies, Inc., and Meridian Bioscience, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Dynamics
Market Driver - Increasing incidence of monkeypox cases globally
The rapid global rise in monkeypox (Mpox) cases is fueling the demand for monkeypox diagnostics. For Instance, on August 14, 2024, the WHO declared the monkeypox outbreak in the Democratic Republic of the Congo (DRC) and other African nations a Public Health Emergency of International Concern (PHEIC), underscoring the critical need to address the escalating outbreak.
The spread of a new strain (clade 1b) has led WHO to enhance support, expand vaccine access, and call for international collaboration.
Market Challenge - High costs associated with advanced diagnostic tests
The global monkeypox (Mpox) diagnosis market faces significant restraints due to high costs associated with advanced diagnostic tests. These expenses limit accessibility, particularly in low-resource settings, hindering timely diagnosis and treatment. Additionally, the complexity of these tests often requires specialized personnel and infrastructure, further escalating costs. As a result, many healthcare facilities may opt for less accurate, cost-effective alternatives, which can delay effective disease management and control efforts in affected regions.
Market Opportunity - Rising demand for rapid and accurate testing solutions
The global monkeypox (Mpox) diagnosis market is growing due to increasing outbreaks and the demand for rapid, accurate tests. Conventional methods are too slow, driving interest in rapid point-of-care tests using platforms like lateral flow assays, CRISPR, and portable polymerase chain reaction. Integrated devices for non-laboratory settings are also gaining popularity. Collaborations with research institutes and government funding are accelerating the development of advanced tests, fueling market growth and expected significant revenue increases in the coming years.
Market Segmentation
- By Test Type Insights (Revenue, USD Bn, 2020 - 2032)
-
- Polymerase Chain Reaction (PCR) Tests
- Serological Tests
- Antigen Detection Tests
- Viral Culture Tests
- By Mode Insights (Revenue, USD Bn, 2020 - 2032)
-
- Laboratory Testing
- Point of Care Testing
- By Component Insights (Revenue, USD Bn, 2020 - 2032)
-
- Hardware
- Software
- By End User Insights (Revenue, USD Bn, 2020 - 2032)
-
- Hospitals and Clinics
- Diagnostic Laboratories
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
-
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
-
- Aegis Sciences Corporation
- Laboratory Corporation of America Holdings
- Quest Diagnostics Incorporated
- Sonic Healthcare Limited
- QIAGEN
- Mayo Clinic Laboratories
- Chembio Diagnostics, Inc.
- Sonora Quest Laboratories
- Thermo Fisher Scientific
- BD (Becton, Dickinson and Company)
- CerTest Biotech
- GenMark Diagnostics, Inc.
- Roche Diagnostics
- Abbott Laboratories
- Hologic, Inc.
- Siemens Healthineers
- PerkinElmer, Inc.
- Bio-Rad Laboratories, Inc.
- Agilent Technologies, Inc.
- Meridian Bioscience, Inc.
Share
Share
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
