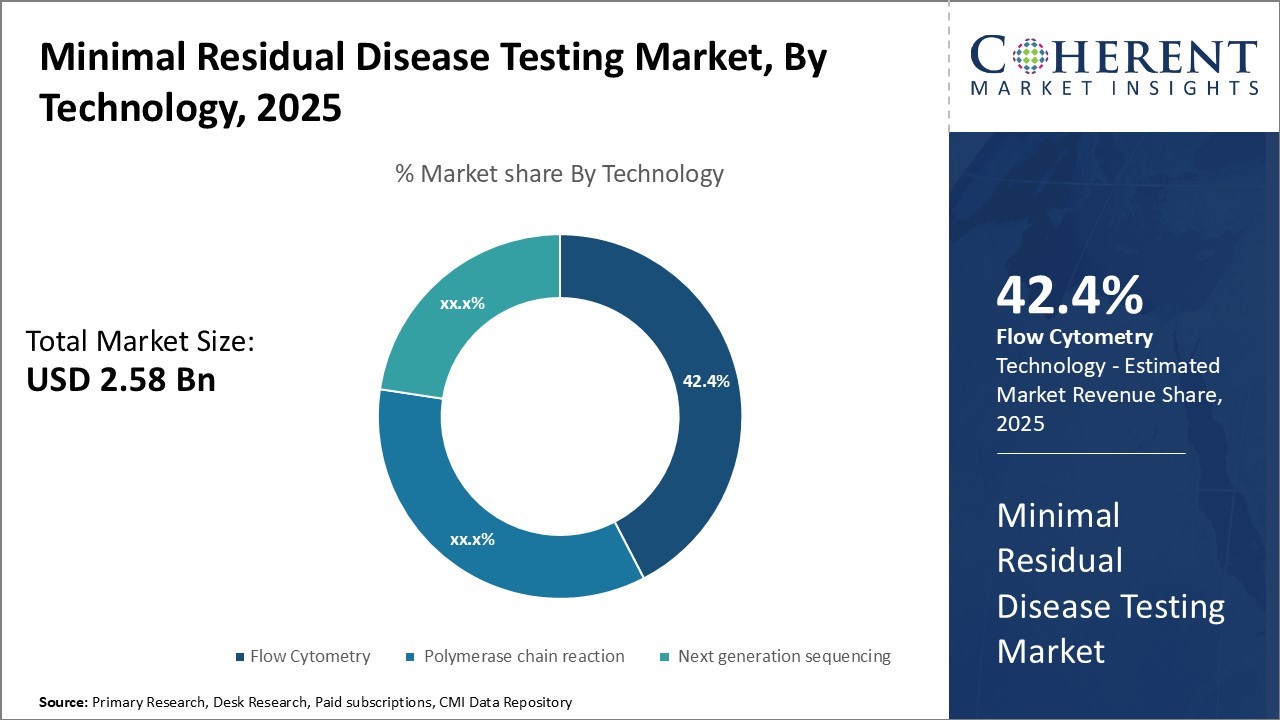
Minimal Residual Disease Testing Market Size and Trends
The minimal residual disease testing market is estimated to be valued at USD 2.58 Bn in 2025 and is expected to reach USD 5.64 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 11.8% from 2025 to 2032.
Key Takeaways
- Based on Technology, the Flow Cytometry segment is expected to account for 42. 4% of the market in 2025, driven by its unmatched sensitivity in detecting rare tumor cells at very low concentrations.
- Based on Application, the Lymphoma segment is projected to hold a 51. 8% share of the market in 2025, mainly driven by their clinical need to closely monitor the treatment response and relapse risk in cancer.
- Based on End User, the Hospital segment is expected to represent 37. 4% of the market in 2025, fueled by institutional infrastructure advantages.
- Based on Region, North America is expected to lead the minimal residual disease testing market with an estimated 35. 0% share in 2025, driven by the presence of top pharmaceutical and biotechnology companies across the United States. While, Asia Pacific is considered to be the fastest growing region during the forecast period due to improved healthcare infrastructure.
To learn more about this report, Download Free Sample
Market Overview
The minimal residual disease testing market is expected to witness significant growth over the forecast period. This can be primarily attributed to rising cancer prevalence worldwide coupled with increasing adoption of minimal residual disease testing in healthcare facilities for accurate prognosis prediction. MRD technologies enable detection of residual tumor cells which helps determine appropriate treatment approach and chance of relapse. With benefits such as real-time disease monitoring and treatment optimization, the demand for MRD tests is expected to surge substantially in the coming years. Additionally, ongoing efforts by market players to develop highly sensitive technologies will further aid the market expansion through the forecast period.
Current Events and their Impact on the Minimal Residue Disease Testing Market
|
Current Event |
Description and its Impact |
|
Regulatory Developments Impacting Market Access |
|
|
Technological Advancements Reshaping Testing Capabilities |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement Scenario in Minimal Residual Disease
Minimal Residual Disease (MRD) testing has gained significant traction in oncology, with several assays now reimbursed by Medicare in the United States. These reimbursements are primarily managed through the Centers for Medicare & Medicaid Services’ (CMS) Molecular Diagnostic Services Program (MolDX), which evaluates and determines coverage for advanced diagnostic tests.
clonoSEQ® by Adaptive Biotechnologies
- Coverage: Approved for MRD assessment in multiple hematologic malignancies, including multiple myeloma, chronic lymphocytic leukemia, B-cell acute lymphoblastic leukemia, mantle cell lymphoma, and diffuse large B-cell lymphoma.
- Reimbursement: As of January 1, 2025, the Medicare Clinical Laboratory Fee Schedule (CLFS) rate for clonoSEQ is set at $2,007. This rate was determined through the gapfill process, where CMS evaluates the test’s value based on factors such as resources required, rates paid by other payers, and unique attributes of the test.
RaDaR® by NeoGenomics
- Coverage: Covered for patients with hormone receptor-positive, HER2-negative breast cancer, including those with a personal history of high-risk stage II/III disease, five or more years from diagnosis, who presently do not have evidence of disease.
- Reimbursement: Coverage was granted by Palmetto GBA, a Medicare Administrative Contractor overseeing the MolDX program, effective March 24, 2023.
Minimal Residual Disease Testing Market Insights, By Technology
Flow Cytometry's Holds the Dominant Share
In terms of technology, the flow cytometry segment is estimated to hold 42.4% share of the market in 2025 owing to its unmatched sensitivity in detecting rare tumor cells at very low concentrations. Flow cytometry relies on fluorescent dye or antibody labelling of cells to uniquely identify malignant cells present at frequencies as low as one in a million. This extreme sensitivity enables detection of minimal residual disease that may exist post-treatment and enable early identification of relapse risk. The ability to sensitively detect rare cancer cells circulating in blood or bone marrow aspirates at the single cell level is crucial for monitoring treatment response and tailoring further therapy. The technology's multi-parameter analysis capabilities also allow identification of multiple tumor-associated markers simultaneously, enhancing accuracy. This multi-dimensional analysis differentiates malignant cells from normal cells based on unique expression profiles detected across several markers. The heterogeneity of blood and tissue samples poses challenges for PCR and NGS methods, but flow cytometry can efficiently sort through complex sample matrices to pinpoint rare tumor populations. Advances in instrumentation have increased throughput while maintaining exquisite analytical sensitivity. Overall, flow cytometry remains the gold standard for minimal residual disease testing due to its unparalleled single cell sensitivity and specificity for detection of rare tumor populations.
In May 2023, Sysmex Corporation unveiled its XF‑1600 Clinical Flow Cytometry System along with the PS‑10 Sample Preparation System and corresponding antibody reagents in Japan, following earlier launches across North America, Europe, and Asia Pacific. Automating the entire diagnostic workflow—from sample prep to analysis, these innovations aim to enhance efficiency and standardize testing for hematologic conditions such as leukemia and lymphoma, further positively influencing the minimal residential disease testing market forecast.
Minimal Residual Disease Testing Market Insights, By Application
Lymphoma Monitoring Relies on MRD Testing
In terms of application, the lymphoma segment is estimated to hold 51.8% share of the market in 2025 due to the clinical need to closely monitor the treatment response and relapse risk in these cancers. Lymphoma includes both Hodgkin's and non-Hodgkin's subtypes that are generally treated via chemotherapy with or without stem cell transplantation. Achieving a complete response to initial therapy is a strong prognostic indicator, but minimal residual disease may persist in some patients and subsequently cause relapse. MRD testing provides crucial post-treatment surveillance for lymphomas, as it can identify patients who are not fully cleared of disease and are at higher risk. This enables preemptive interventions such as adjustment of maintenance therapies or closer follow up monitoring.
In January 2025, Immuneel Therapeutics, backed by biotech leader Kiran Mazumdar-Shaw, introduced Qartemi, India’s first globally benchmarked CAR T‑cell therapy targeting adult B‑cell non‑Hodgkin lymphoma (B‑NHL). Qartemi uses patients’ own genetically engineered T‑cells to combat refractory cancer types, showing an 83.3% overall response rate in phase II IMAGINE trials across leading centers including Narayana Hospital, Apollo Cancer Hospital, and PGIMER.
Minimal Residual Disease Testing Market Insights, By End User
Hospitals Lead MRD Testing due to Infrastructure
In terms of end user, the hospital subsegment is expected to have 37.4% of the market share in 2025 owing to institutional infrastructure advantages. MRD testing requires specialized laboratory equipment, trained technicians, and multi-disciplinary collaboration between pathology, hematology and oncology specialties. Most hospitals have centralized pathology labs with flow cytometers, PCR machines and the capacity to perform complex genomic analyses. This efficient concentration of analyzers and expertise enables high volume, standardized testing.
Additionally, hospitals treat the majority of cancer patients receiving chemotherapy or stem cell transplants. This consolidates sample collection locally and facilitates utilizing MRD status to guide clinical management and follow up care within the same organization. Hospitals also employ hematopathologists and oncologists who can properly select patients for MRD monitoring, integrate results into patient care, and conduct further translational research. Commercialization of testing services has emerged but remains supplementary to hospital laboratories forming the core infrastructure. Efforts to decentralize testing to community clinics and physician offices are limited by complex assay requirements. As a result, hospitals play the lead role in fulfilling clinical needs for minimal residual disease monitoring, thereby proliferating the minimal residue disease testing market revenue.
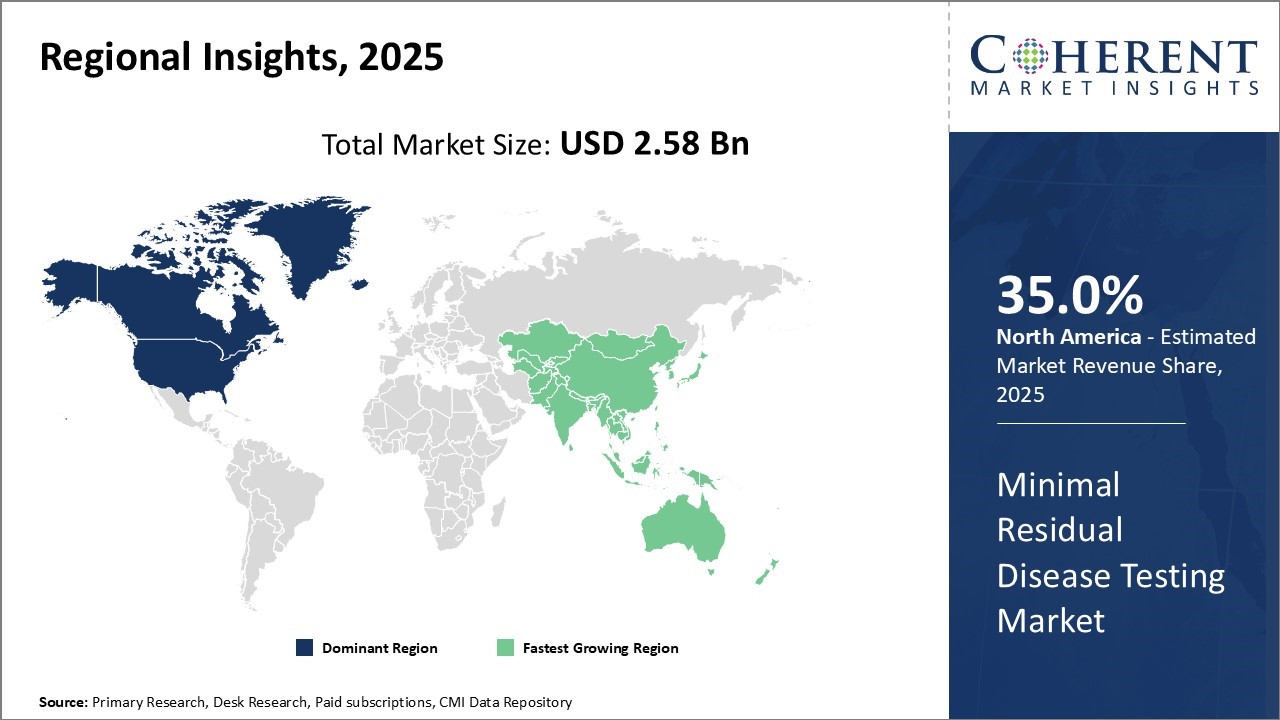
Regional Insights
To learn more about this report, Download Free Sample
North America Minimal Residual Disease Testing Market Analysis & Trends
North America remains the dominant region in the global minimal residual disease testing market and is anticipated to hold 35.0% of the market share in 2025. North America has established itself as the leading region in the global minimal residual disease testing market. The presence of some of the top pharmaceutical and biotechnology companies along with advanced healthcare infrastructure and high healthcare spending have fueled market growth. The U.S. accounts for the largest share due to the growing adoption of MRD testing in clinical trials and patient monitoring practices. Several hospitals and laboratories in the country are well-equipped with modern testing technologies and skilled professionals to perform these tests routinely.
In Canada, minimal residual disease (MRD) testing is performed using flow cytometry and is funded through hospital or laboratory budgets in British Columbia, Nova Scotia, and Manitoba.
Asia Pacific Minimal Residual Disease Testing Market Analysis & Trends
Asia Pacific is recognized as the fastest-growing market for MRD testing globally. The growth can be attributed to improving healthcare infrastructure, expanding patient pool of hematological cancers, and growing medical tourism across India, China, and other countries. Various initiatives by governments to promote localization of production are also encouraging international companies to tap into opportunities. Japan saw a 38% increase in molecular diagnostics, while South Korea recorded approximately 85,000 MRD tests. This is further accelerating the minimal residue disease testing market share.
Minimal Residual Disease Testing Market Outlook Country-Wise
The United States Minimal Residual Disease Testing Market Trends
The U.S. minimal residual disease testing market is driven by the high rate of hematological malignancies in the U.S. According to the Leukemia & lymphoma Society, it is estimated that, there are 187,740 people were diagnosed with hematological malignancies (leukemia, lymphoma, or myeloma) in 2024. Additionally, over 1.6 million individuals are living with or in remission from these cancers, including myelodysplastic syndromes (MDS) and myeloproliferative neoplasms (MPNs). Along with that, the U.S. has a robust reimbursement structure based on insurance type, but generally, most comprehensive health plans cover cancer treatment, including chemotherapy, radiation therapy, surgery, and related services.
China Minimal Residual Disease Testing Market Trends
China’s minimal residual disease testing market has seen notable growth due to the high cancer burden, advancements in biotechnology, and increased investment in healthcare infrastructure. The country's focus on MRD testing is driven by the need for more effective strategies to manage and monitor diseases like leukemia, lymphoma, and multiple myeloma. According to Chinese Medical Journal, in 2024, there are ae estimated new cancer cases and 1,699,066 cancer deaths in China. The highest estimated cancer cases are lung cancer in China.
In June 2025, Chinese biotech firms has made a total around $25 billion in upfront and milestone payments—aiming to access cutting-edge cancer therapies, particularly PD‑1/VEGF bispecific antibodies. These agreements, including notably large investments from Pfizer and Bristol Myers Squibb, signal confidence in China's growing biotech innovation, even as Washington contemplates tariffs on biotech imports.
Germany Minimal Residual Disease Testing Market Trends
Germany remains a leader in minimal residual disease testing market, known for a combination of factors including strong academic and research institutions, well-established clinical guidelines, and a focus on optimizing treatment strategies based on MRD status. The GMALL (German Multicenter Study Group for Adult ALL) plays a significant role in evaluating MRD and MRD-based treatment decisions in adult acute lymphoblastic leukemia (ALL), contributing to the understanding of MRD as a prognostic factor. For instance, In January 2025, Datar Cancer Genetics launched Target-MRD, an advanced blood test for monitoring minimal residual disease (MRD) in solid organ cancers in Germany.
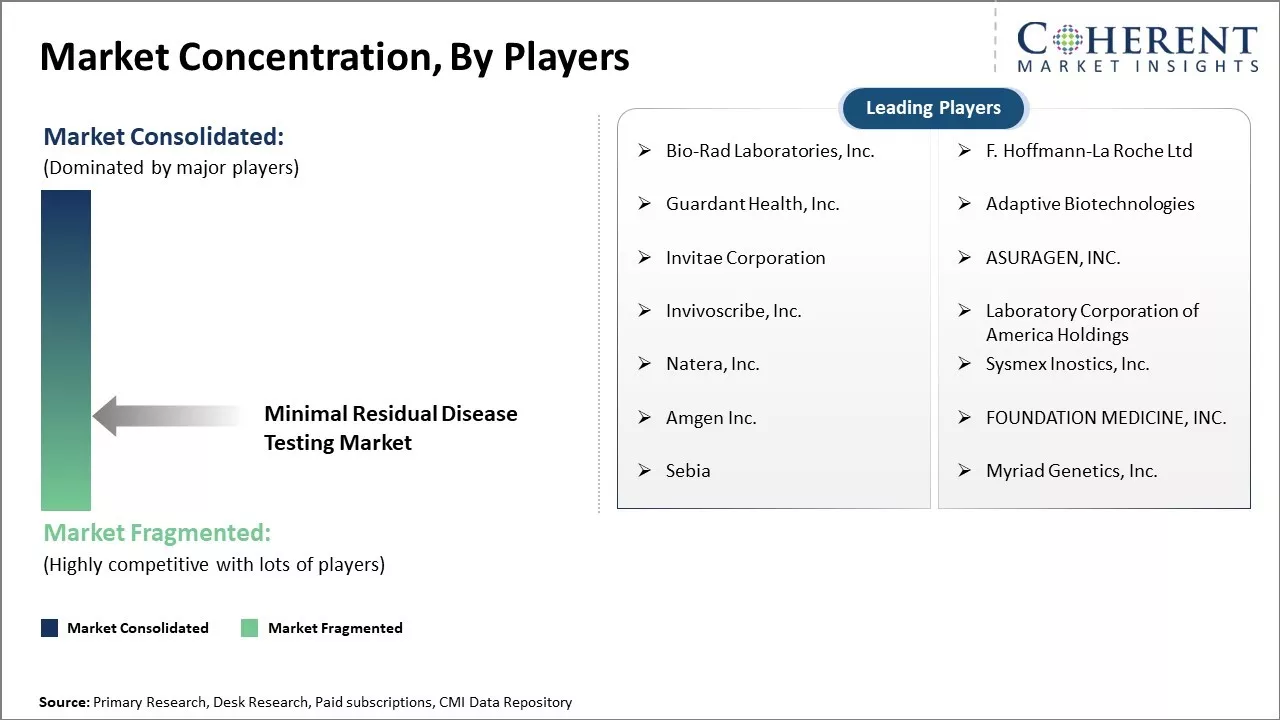
Market Concentration and Competitive Landscape
To learn more about this report, Download Free Sample
Minimal Residual Disease Testing Industry News
- In June 2025, QIAGEN expanded its minimal residual disease (MRD) testing capabilities through two strategic partnerships, enhancing its oncology diagnostics portfolio. Firstly, QIAGEN and Tracer Biotechnologies will co-develop blood-based MRD assays for solid tumors on the QIAcuity digital PCR platform, enabling high-sensitivity cancer monitoring using minimally invasive samples. Secondly, a collaboration with Foresight Diagnostics aims to commercialize a kit-based version of the “CLARITY™ NGS assay” for lymphoma, transitioning ctDNA testing from centralized labs to decentralized settings.
- In January 2025, Datar Cancer Genetics (DCG) has unveiled Target‑MRD, a cutting-edge blood test designed to detect and monitor molecular residual disease (MRD) across solid organ cancers. The test uniquely combines tumor-informed ddPCR and tumor-agnostic NGS to deliver ultra-sensitive detection—even at extremely low disease burdens—enabling physicians to identify potential relapse earlier than conventional genomic or imaging methods.
- In January 2025, Exact Sciences Corp. has released compelling new results demonstrating its Oncodetect™ molecular residual disease (MRD) test effectively predicts cancer recurrence. Findings from the Alpha-CORRECT study, presented at the 2025 ASCO GI Symposium, showed 78% sensitivity shortly after surgery and 91% during surveillance in stage III colorectal cancer patients, with specificities of 80% and 94%, respectively.
- In June 2024, SOPHiA GENETICS, a leader in cloud-native healthcare tech, has announced the launch of its new Residual Acute Myeloid (RAM) application, expanding its oncology suite with cutting-edge measurable residual disease (MRD) capability. It is designed to support post-remission monitoring in Acute Myeloid Leukemia (AML) patients—who face relapse rates exceeding 50% within three years, the RAM tool leverages next-generation sequencing to detect as few as one cancer cell among 10,000, with sensitivity down to 0.01% variant allele frequency.
Market Report Scope
Minimal Residual Disease Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.58 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.8% | 2032 Value Projection: | USD 5.64 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Bio-Rad Laboratories, Inc., F. Hoffmann-La Roche Ltd, Guardant Health, Inc., Adaptive Biotechnologies, Invitae Corporation, ASURAGEN, INC., Invivoscribe, Inc., Laboratory Corporation of America Holdings, Natera, Inc., Sysmex Inostics, Inc., Amgen Inc., FOUNDATION MEDICINE, INC., Sebia, and Myriad Genetics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Minimal Residual Disease Testing Market Driver
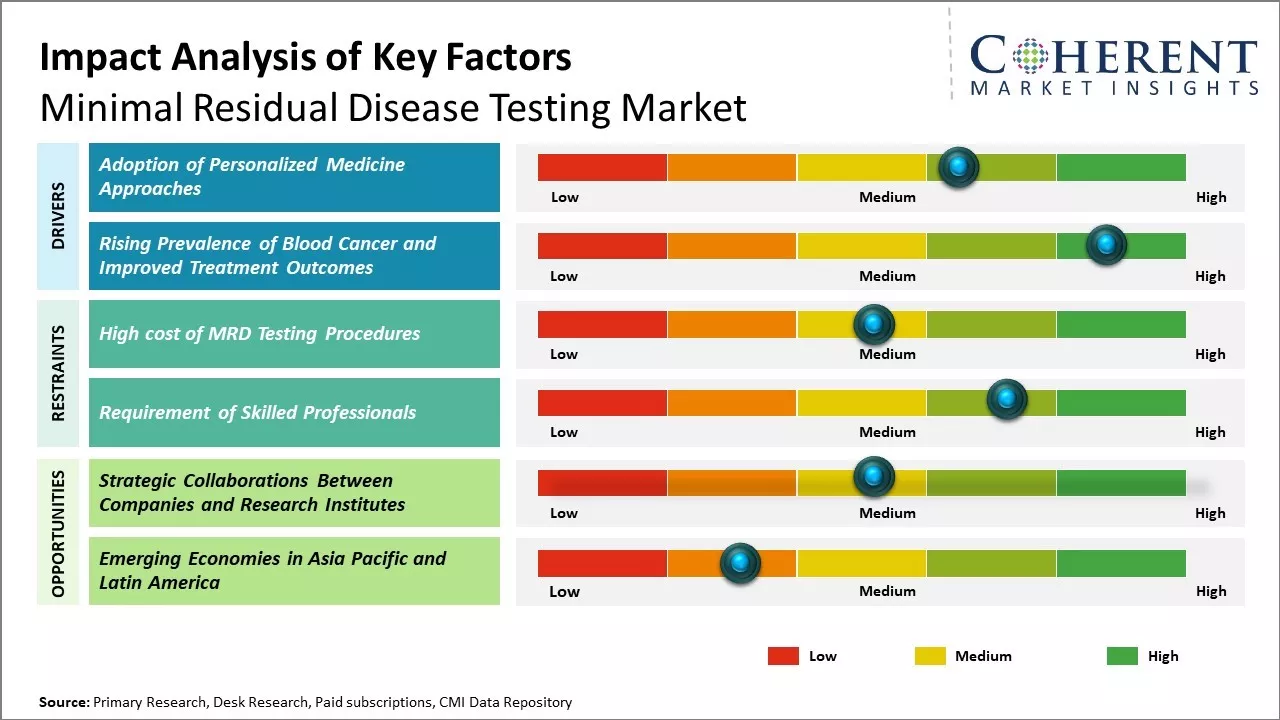
Rising Prevalence of Blood Cancer and Improved Treatment Outcomes
The increasing incidence and prevalence of various types of hematological malignancies including leukemia, lymphoma, and multiple myeloma across the world has been a major factor fueling the demand for minimal residual disease testing. According to key opinion leaders, the early and accurate detection of residual cancer cells after initial treatment is critical in blood cancers in order to assess treatment response and make adjustments to therapy if needed. MRD testing allows physicians to closely monitor patients and customize their treatment approach. For instance, in August 2022, according to data published by Blood Cancer UK Research, it was stated that blood cancer is the fifth most common cancer in the U.K., with over 41,000 people being diagnosed with it every year. With advancements in treatment options such as targeted therapies, immunotherapy, and stem cell transplantation, more patients are achieving remission and long-term survival.
However, the risk of relapse persists if residual cancer cells remain present even at low levels after primary treatment. MRD testing helps identify such residual disease that may not be detected by conventional imaging and ensures patients receive timely and appropriate intervention such as additional chemotherapy cycles or stem cell transplant. This is expected to improve long term outcomes. The ability of MRD to predict relapse and guide treatment strategies non-invasively without the need for invasive biopsies provides a significant advantage. With growing success rates of initial treatments, monitoring of MRD will become an important tool helping clinicians effectively manage blood cancer patients over the long term, propelling the minimal residue disease testing market share.
Adoption of Personalized Medicine Approaches
There is a growing trend in the oncology field towards more personalized and precision medicine based on a patient’s specific disease characteristics and risk factors. Advanced MRD technologies allow the quantitative assessment of the tumor burden right down to a single cancer cell. Key opinion leaders note that MRD status is already used to personalize post-remission therapy decisions in some blood cancer types. For example, in acute myeloid leukemia, an MRD negative status may allow stopping further treatment whereas MRD positivity could warrant a stem cell transplant. Researchers are also exploring use of MRD for predicting survival outcomes and tailoring surveillance schedules accordingly. As clinical studies establish MRD as a reliable biomarker, its use for personalized risk stratification and subsequent personalization of treatment regimens is expected to increase tremendously. This drives the need for the wider adoption of sensitive and standardized MRD testing methods across the continuum of care.
Minimal Residual Disease Testing Opportunities
Strategic Collaborations Between Companies and Research Institutes
Strategic collaborations between companies and research institutes could be a great opportunity in the minimal residual disease testing market. When companies partner with academic institutions and leverage their research expertise, it helps accelerate the development of novel and improved testing technologies. For instance, in June 2023, Myriad Genetics, Inc., a biotechnology company, announced a collaboration agreement with The University of Texas MD Anderson Cancer Center to support research focused on metastatic renal cell carcinoma treatment selection and response. The project will use Myriad Genetics, Inc.’s minimal residual disease (MRD) testing platform, a tumor-informed high-definition assay that detects circulating tumor DNA (ctDNA).
Analyst Opinion (Expert Opinion)
- The Minimal Residual Disease (MRD) testing market is on the cusp of clinical and technological realignment—driven not by volume but by precision, timing, and therapeutic consequence. What makes MRD testing disruptive is its ability to decisively challenge the traditional reliance on morphological assessments and post-treatment imaging. We are no longer discussing if MRD will become central to hematologic malignancy care, but how fast it will displace legacy modalities.
- In multiple myeloma, studies have shown that MRD negativity correlates more strongly with progression-free survival (PFS) than complete response (CR) by conventional metrics. For instance, the IFM 2009 trial revealed that MRD-negative patients had a median PFS of 56.4 months versus 29.7 months for MRD-positive counterparts—even when both groups met the same clinical remission criteria. That is a paradigm shift.
- MRD is now an active decision trigger. In Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL), MRD positivity post-induction therapy routinely dictates whether a patient moves toward allogeneic stem cell transplantation or remains on TKI therapy. This transforms MRD from a retrospective marker to a prospective roadmap.
- Regulatory momentum is also lending MRD institutional gravity. The FDA’s approval of clonoSEQ® as the first and only assay for MRD assessment in lymphoid malignancies was not just a clinical endorsement—it signaled a market transition toward standardized, quantitative, molecular diagnostics. The fact that MRD is now being accepted as a surrogate endpoint in clinical trials (e.g., in CLL, MM, and ALL) underscores how drug development is being restructured around MRD status.
- The battleground is between next-generation sequencing (NGS) and flow cytometry. While flow remains cost-effective and widely adopted, its ceiling is evident—it cannot match the 10^-6 sensitivity of NGS. Adaptive Biotechnologies’ NGS-based clonoSEQ platform detects one cancer cell among a million healthy cells. In contrast, even optimized multi-parameter flow cytometry caps out at 10^-5. That one-log difference is not just academic—it determines whether relapses are caught in time or not.
- Moreover, as personalized cell therapies (like CAR-T) gain traction, MRD testing will become essential not only for pre-treatment stratification but for post-infusion surveillance. Without high-fidelity MRD, the clinical efficacy of precision immunotherapies becomes a black box. Several CAR-T trials already use MRD negativity as a primary response metric rather than traditional complete response.
Market Segmentation
- Technology Insights
- Flow Cytometry
- Polymerase chain reaction (PCR)
- Next generation sequencing
- Application Insights
- Lymphoma
- Solid Tumors
- Others
- End User Insights
- Hospitals
- Clinics
- Research Laboratories
- Others
- Regional Insights
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Bio-Rad Laboratories, Inc.
- F. Hoffmann-La Roche Ltd.
- Guardant Health, Inc.
- Adaptive Biotechnologies
- Invitae Corporation
- ASURAGEN, INC.
- Invivoscribe, Inc.
- Laboratory Corporation of America Holdings
- Natera, Inc.
- Sysmex Inostics, Inc.
- Amgen Inc.
- FOUNDATION MEDICINE, INC.
- Sebia
- Myriad Genetics, Inc.
Sources
Primary Research Interviews from the following stakeholders
Stakeholders
- Interviews with hematologists, oncologists, lab directors, molecular diagnostics heads, bioinformatics professionals, pathology lab managers, and procurement leads across major cancer care and research centers globally.
Specific stakeholders
- Clinical leads in oncology at cancer research institutes
- Diagnostic lab managers at national pathology chains
- Molecular diagnostics heads at multispecialty hospitals and private care networks
- Academic researchers involved in MRD assay validation
- IT and LIMS administrators handling genomic data integration
- Procurement heads for diagnostic equipment and consumables at hospital groups
- Clinical trial managers at CROs and pharma-biotech firms focused on hematologic cancers
Databases
- Global Burden of Disease (GBD) Database
- National Cancer Registry Programme (NCRP), India
- SEER Program
- EU Clinical Trials Register
- ClinicalTrials.gov
- Japan Cancer Information Service (JCIS)
- World Health Organization (WHO)
- Cancer Research UK
- Central Drugs Standard Control Organization (CDSCO), India
- U.S. Food and Drug Administration (FDA)
Magazines
- Cancer World
- Oncology Times
- Genetic Engineering & Biotechnology News (GEN)
- Clinical Lab Products Magazine
- Diagnostics World
- Lab Manager
- The Pathologist
- Medtech Insight
- Modern Healthcare – Diagnostics Section
- BioSpectrum
Journals
- Blood (American Society of Hematology)
- Leukemia (Nature Publishing)
- Journal of Clinical Oncology (ASCO)
- Clinical Chemistry (AACC)
- Journal of Molecular Diagnostics
- Cancer Medicine (Wiley)
- Annals of Hematology
- NPJ Precision Oncology
- Frontiers in Oncology – Hematological Malignancies
- Journal of Hematology & Oncology
Newspapers
- The Economic Times – Healthcare & Diagnostics
- Financial Express – Pharma and Biotech Section
- Stat News – Oncology and Diagnostics Coverage
- The Hindu – Science & Health
- Business Standard – Life Sciences Segment
- The New York Times – Health and Science
- Reuters Health
- Nature News – Cancer and Lab Technologies
- Washington Post – Precision Medicine Coverage
Associations
- American Society of Hematology (ASH)
- European Hematology Association (EHA)
- College of American Pathologists (CAP)
- American Association for Clinical Chemistry (AACC)
- Association for Molecular Pathology (AMP)
- Indian Society of Hematology and Blood Transfusion (ISHBT)
- National Comprehensive Cancer Network (NCCN)
- Federation of Indian Chambers of Commerce and Industry (FICCI) – Lifesciences Division
- European Society for Medical Oncology (ESMO)
- International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Public Domain Sources
- Indian Council of Medical Research (ICMR)
- Ministry of Health and Family Welfare (MoHFW), India
- National Cancer Institute (NCI), U.S.
- European Medicines Agency (EMA)
- Department of Biotechnology (DBT), India
- National Institutes of Health (NIH), U.S.
- Biotechnology Industry Research Assistance Council (BIRAC)
- U.K. National Health Service (NHS) – Genomics England
- Health Canada – Medical Devices & Diagnostics Division
- National Health Authority (NHA) – Digital Health Data Reports
Proprietary Elements
- CMI Data Analytics Tool, and Proprietary CMI Existing Repository of information for last 8 years
*Definition: Minimal residual disease (MRD) testing is a highly sensitive method for detecting cancer cells in patients with blood cancers such as myeloma, lymphoma, and leukemia. MRD testing is used to determine if cancer cells have been killed by chemotherapy or other cancer treatments. It can help determine if a patient is in full remission, at risk of relapse, or if further treatment is needed.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients