Medical Engineered Materials Market is estimated to be valued at USD 31.58 Bn in 2025 and is expected to reach USD 75.69 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 13.3% from 2025 to 2032.
Global medical engineered materials market is segmented into by product type, application, end user, and region. By product type, the market is segmented into metallic biomaterials, ceramics biomaterials, polymeric biomaterials, natural biomaterials, and composites biomaterials. Polymeric biomaterials accounted for the largest share in 2023. The large share of this segment can be attributed to the various advantages offered by polymeric biomaterials such as biocompatibility, flexibility, and easy manufacturability.
Global Medical Engineered Materials Market- Regional Insights
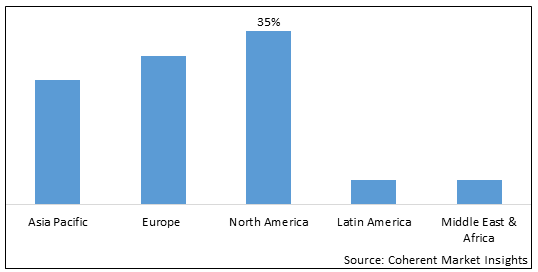
- North America is expected to be the largest market for medical engineered materials during the forecast period, accounting for over 35.2% of the market share in 2025. The growth of the market in North America is attributed to rising prevalence of chronic diseases, growing geriatric population, and increasing adoption of advanced biomaterials.
- Asia Pacific is expected to be the second-largest market for medical engineered materials t, accounting for over 25.5% of the market share in 2025. The growth of the market in is attributed to the expanding healthcare sector, large patient pool, and increasing healthcare expenditure in the region.
- Europe is expected to be the fastest-growing market for medical engineered materials, with a CAGR of over 27.1% during the forecast period. The growth of the market in Europe is attributed to the presence of leading medical device manufacturers, favorable government policies, and growing R&D activities related to engineered biomaterials in the region.
Figure 1. Global Medical Engineered Materials Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Medical Engineered Materials Market- Drivers
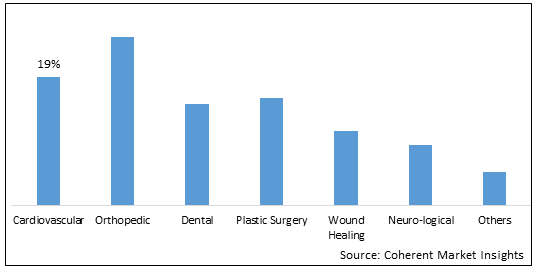
- Growing prevalence of chronic diseases: Rising prevalence of chronic diseases such as cardiovascular diseases, neurological disorders, orthopedic conditions, and cancers is a major factor driving the global medical engineered materials market. Chronic diseases are on the rise globally due to sedentary lifestyles, unhealthy diets, pollution, and higher life expectancy. Engineered biomaterials are increasingly used for treatment and management of various chronic disorders. For instance, cardiovascular stents made of metallic alloys improve blood circulation in blocked arteries. Polymeric materials are used to develop drug delivery systems for targeted therapy of cancer. The demand for such engineered biomaterials is expected to grow as the burden of chronic diseases increases globally.
- Technological advancements in engineered biomaterials: Significant advances made in material science and engineering technologies are facilitating development of novel engineered biomaterials with enhanced properties and performance. Technologies like 3D printing/additive manufacturing allow fabrication of intricate designs and customized engineered biomaterials suitable for patient-specific needs. Nanotechnology is enabling engineering of nano-scale biomaterials with superior mechanical, electrical and biological properties. Tissue engineering has led to development of scaffolds using biomaterials that can regenerate damaged tissues and organs. Such technological innovations are creating new growth avenues for engineered biomaterials in treatments like regenerative medicine, biosensors, bioprinting of organs and implants. For instance, according to the U.S. Environmental Protection Agency, municipal solid waste generation had increased by over 4% from 235 million tons in 2018 to 245 million tons in 2020, necessitating sustainable biomaterial alternatives.
- Growth in aging population: Growing geriatric population worldwide susceptible to various age-related disorders is expected to boost demand for medical engineered materials. Older people are more likely to develop chronic illnesses like arthritis, cardiovascular diseases, neurodegenerative diseases, vision/hearing loss, osteoporosis, and others. These conditions require engineered biomaterials like strong lightweight prosthetics, artificial joints, dental implants, cochlear implants and intraocular lenses for treatment. Moreover, engineered scaffolds and matrices stimulate tissue regeneration in the elderly. Rising geriatric population is likely to propel the growth of the market in the near future.
- Investments and research in engineered biomaterials: Increasing investments by public and private players in research activities for development of novel engineered biomaterials for medical use is poised to fuel the market growth. Various academic and research institutes are undertaking studies to engineer advanced biomaterials with desired mechanical properties, biocompatibility and functionality. Moreover, leading medical device and pharmaceutical companies are channelizing funds to establish R&D centers focused on biomaterials research. Partnerships between academia and industry players are also increasing to translate innovative research into commercially viable engineered biomaterials, thereby, offering growth opportunities for the market.
Global Medical Engineered Materials Market- Opportunities
- Applications in personalized medicine: The emerging field of personalized medicine presents significant growth opportunities for engineered biomaterials. Customized biomaterials that match the specific genetic makeup of patients can lead to improved therapeutic outcomes. 3D bioprinting technology enables fabrication of patient-specific organs, tissues and devices using tailored biomaterials and cells sourced from the patients themselves. Companies are also developing drug delivery systems containing engineered biomaterials that can release medicines at a rate aligned to the patient's physiological conditions. Such innovations in precision medicine are creating prospects for engineered biomaterials. For instance, according to National Cancer Institute, the percentage of cancers with a driver mutation identified through genomic profiling will increase from 50% in 2020 to 70% by 2023.
- Rising demand in developing regions: Developing regions worldwide exhibit huge demand potential for medical engineered materials due to improving healthcare infrastructure and increasing healthcare spending. Emerging economies like China, India, Brazil, Mexico, Indonesia, and others have a high patient pool and rising incidence of chronic and lifestyle diseases that require interventions using engineered biomaterials. Local players as well as leading medical device companies are expanding their manufacturing facilities in these regions. Moreover, growing middle class populations, adoption of advanced technologies, favorable government initiatives and policies are key factors likely to serve the market growth in developing regions. For instance, according to United Nations Population Division, the middle class population in India will grow over three times to about 800 million people by 2030, providing a sizable customer segment for medical devices.
- Shifting focus towards bio-based materials: Increasing environmental concerns associated with traditional engineered biomaterials derived from non-renewable sources is steering research efforts towards development of sustainable bio-based materials. Naturally derived biomaterials from marine organisms, microbial sources, agricultural waste, food waste, and others are gaining interest. Biopolymers like collagen, chitosan, silk fibroin, cellulose, starch, and others are being explored for tissue engineering and drug delivery. Bio-based composites mimicking the structure and function of native extracellular matrix are also being developed. The shift towards such renewable and eco-friendly biomaterials is paving new avenues in the market.
- Widening applications in diagnostics: Engineered biomaterials exhibit tremendous scope for use in diagnostic platforms like biosensors, lab-on-chip devices, microfluidic chips, and others due to their biocompatibility, versatility in modifications and tuneable physical/chemical properties. For instance, quantum dots, hydrogels, nanocomposites and conductive polymers are being engineered into platforms for rapid diagnostics, home testing kits, wearable sensors, and others. Companies are also developing injectable nanosensors made from biomaterials for real-time monitoring of biomarkers. Thus, potential use of engineered biomaterials in point-of-care and molecular diagnostics is creating lucrative prospects.
Global Medical Engineered Materials Market- Trends
- Rising adoption of 3D printing: The market is significantly benefitting from the rapid adoption of 3D printing or additive manufacturing technologies for fabrication of intricate and tailor-made engineered biomaterials, implants, prosthetics and organ models. 3D bioprinting in particular facilitates precise deposition of biomaterials and living cells layer by layer to create complex tissues and organs. Companies are leveraging 3D printing to develop patient-specific implants and devices based on their body’s structure. Customization and on-demand manufacturing abilities are key factors driving integration of 3D printing in engineered biomaterials and expanding its applications. For instance, according to reports by the Food and Drug Administration, the number of medical devices and materials receiving marketing authorization after 3D printing rose over 30% from 2020 to 2021. While still a small fraction of overall clearances, it indicates the great potential of this innovative technology.
- Increasing demand for nanomaterials: Nanotechnology-enabled engineering of biomaterials at molecular level is gaining importance, owing to the unique functionalities achieved at nanoscale. Nanostructured materials mimic the natural nanofeatures of tissues down to cellular and molecular hierarchy. Nanoscale modification of biomaterials enhances their mechanical, electrical and biological properties. For instance, nanocomposites with reinforced polymer matrices exhibit superior strength for bone implants. Nanoporous materials allow controlled and sustained drug delivery. Rising demand for nanocellulose, carbon nanotubes, nanoclays, nanocrystals, and others due to their enhanced physico-chemical, anti-microbial and wound healing properties is driving expansion of the nano-biomaterials market. For instance, according to the World Health Organization, around 1 million people acquire healthcare-associated infections every year globally. The use of nanotechnology could help to address this major problem.
- Advances in tissue engineering scaffolds: Significant progress in fabrication of porous tissue engineering scaffolds using advanced biomaterials is a major trend fostering the market growth. Design of scaffolds with controlled biodegradability, porosity, interconnectivity and optimized surface chemistry is enabling regeneration of tissues. Advances like electrospinning facilitate production of nanofiber and microfiber scaffolds mimicking the extracellular matrix. Companies are incorporating cells, growth factors, peptides, and othersinto scaffolds to create functional living tissues and organs through tissue engineering. Continuous expansion in scaffold design and biofabrication technologies is projecting a positive growth outlook.
- Growing coatings applications: The market is benefitting significantly from rising adoption of engineered biomaterial-based coatings in medical devices to enhance biocompatibility and therapeutic efficacy. For example, anti-microbial coatings containing silver nanoparticles are being used to reduce risk of post-surgical infections associated with implants and indwelling devices. Lubricious hydrogel coatings minimize friction between devices and tissues in procedures like endoscopy. Companies are also developing smart polymer coatings that release drugs in a controlled and targeted manner. Further, bioactive ceramic coatings applied on implants facilitate bone integration. The growing trend of surface modification and functionalization of devices with engineered coatings is fueling the market growth.
Medical Engineered Materials Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 31.58 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.3% | 2032 Value Projection: | USD 75.69 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Evonik Industries AG, Covestro AG, BASF SE, Solvay, SABIC, Trelleborg AB, DSM, Celanese Corporation, DuPont de Nemours Inc |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Medical Engineered Materials Market- Restraints
- Stringent clinical & regulatory requirements: Lengthy and stringent approval processes mandated by regulatory agencies like FDA for newly developed engineered biomaterials poses key challenges for manufacturers in terms of time and costs. Biomaterials must undergo rigorous preclinical and clinical evaluation to establish safety and efficacy before approval for commercialization. Moreover, the regulatory landscape is continuously evolving with changing validation requirements, calling for expensive modifications. The complex and ambiguous regulatory scenario across different geographies acts as a major barrier for uptake of latest engineered biomaterials. For instance, according to the European Commission, between 2020-2022, the EU has invested 297 million US$ in Horizon Europe to support research on advanced therapies and novel materials for implants and prosthetics. While ensuring patient welfare, such initiatives aim to spur growth by helping worthy projects overcome regulatory hurdles more efficiently.
- High development and production costs: High capital requirements for R&D and manufacturing of engineered biomaterials along with long timelines from concept to realization is a major restraint faced by developers and suppliers. Significant investments are required for establishing advanced material engineering facilities, acquiring latest fabrication and characterization technologies, and recruiting skilled researchers. Moreover, scale-up from lab to commercial batches involves substantial expenses. Lack of proper funding resources hampers innovation and adoption of novel engineered biomaterials, thereby hindering the market growth. For instance, in 2022, according to the report published by National Institutes of Health, 3D printing of titanium implants involve specialized multi-step processes like powder production, part fabrication, heat treatment and surface modification which increases the cost by 5-10 times as compared to conventional implants. Similarly, development of new bio absorbable materials require careful tuning of material properties like degradation rate through modifications in chemical composition and microstructure which is a costly trial-and-error process.
- Reimbursement challenges: Limited reimbursement coverage provided by healthcare payers like Medicare, Medicaid and private insurers for treatments involving newer engineered biomaterials poses challenges for market growth. Due to lack of medical evidences from long-term clinical studies, insurers hesitate to provide payment approval for newly introduced biomaterials. Patients are reluctant to adopt treatments using latest biomaterials, owing to high out-of-pocket costs, unless reimbursement policies are improved. Lack of reimbursement infrastructure especially in developing regions negatively impacts the product adoption.
Analyst View
Global medical engineered materials market has strong growth potential over the next five years. Demand for high-performance materials is increasing from the medical devices industry as technologies advance. Population aging globally will boost demand for implants, prosthetics, and other device-based care. New applications in drug delivery, regenerative medicine, and implant coatings present opportunities for materials suppliers to partner with medtech OEMs. Plastic and polymer materials are expected to see heightened adoption as these allow for lower cost and customized solutions. Metal alloy usage may witness declines due to their limitations in customizability. Bioresorbable materials may open up new design possibilities, but more clinical validation is needed to realize their potential. North America and Europe are likely to remain the dominant regions due to the concentration of leading medical professionals and the mature healthcare market. However, Asia Pacific market will witness growth due to expanding medical infrastructure, growing private healthcare, and strong manufacturing competitiveness in countries like China, India, and South Korea. High material and regulatory costs pose challenges, necessitating innovations that reduce the overall cost of care. Success will depend on the ability to address unique performance needs of diverse medical specialties. Overall, the medical engineered materials landscape presents opportunities for suppliers that forge deep partnerships, deliver specialized solutions, and help lower the barriers to advanced healthcare access globally.
Global Medical Engineered Materials Market- Recent Developments
New product launches:
- In 2023, Evonik Industries AG launched a new biocompatible thermoplastic polyurethane (TPU) for medical applications. The material is called "Celstran Bio" and is designed to be used in implants, medical devices, and other applications where biocompatibility is critical. Evonik Industries AG is a publicly-listed Germany-based specialty chemicals company. It is the second-largest chemicals company in Germany, and one of the largest specialty chemicals companies in the world.
- In 2023, CoorsTek launched a new line of medical-grade ceramics. The materials are called "Ceramics for Life," and are designed to be used in a variety of medical applications, including implants, drug delivery devices, and surgical tools. CoorsTek, Inc. is a privately owned manufacturer of technical ceramics for aerospace, automotive, chemical, electronics, medical, metallurgical, oil and gas, semiconductor and many other industries. CoorsTek headquarters and primary factories are located in Golden, Colorado, U.S.
- In 2023, DSM Biomedical launched a new bioresorbable polymer called "Resomer." The material is designed to be used in implants that are designed to dissolve over time. DSM Biomedical provides medical device materials. The company offers adhesive, bioceramics, biomedical polyethylenes and polyurethanes, collagen, and stabilizing technology services. DSM Biomedical serves customers in the United States.
Acquisition and partnerships:
- In March 2023, DSM Biomedical partnered with 3D Systems to develop and commercialize 3D-printed medical devices. The partnership is expected to accelerate the development and adoption of 3D-printed medical devices. DSM Biomedical provides medical device materials. The company offers adhesive, bioceramics, biomedical polyethylenes and polyurethanes, collagen, and stabilizing technology services. DSM Biomedical serves customers in the U.S.
- In February 2023, Covestro AG acquired Heraeus Medical Components, a manufacturer of medical implants. The acquisition is expected to expand Covestro's presence in the medical implants market. Covestro AG is a Germany-based company producing polyurethane and polycarbonate raw materials. Products include isocyanates and polyols for cellular foams, thermoplastic polyurethane and polycarbonate pellets, as well as polyurethane based additives used in the formulation of coatings and adhesives.
- In January 2023, Evonik Industries AG acquired PolyMedix, Inc., a developer of bioresorbable polymers. The acquisition is expected to strengthen Evonik's portfolio of bioresorbable materials for medical applications.
Figure 2. Global Medical Engineered Materials Market Share (%), By Application, 2025
To learn more about this report, Download Free Sample
Top companies in Global Medical Engineered Materials Market:
- Evonik Industries AG
- Covestro AG
- BASF SE
- Solvay
- SABIC
- Trelleborg AB
- DSM
- Celanese Corporation
- DuPont de Nemours Inc.
Definition: Global medical engineered materials market refers to the industry and commercialization of biomaterials synthesized or modified through engineering to interact with biological systems. These biomaterials are used in a variety of medical applications such as implants, prosthetics, drug delivery systems, regenerative medicine, and medical devices to augment or replace biological functions. The engineered materials provide specific chemical, biological, mechanical, and physical properties to match the biological environment. Key types of medical engineered materials include ceramics, metals, polymers, natural materials, and composites.
Few Other Promising Reports in Advanced Materials Industry
Share
Share
About Author
Vidyesh Swar is a seasoned Consultant with a diverse background in market research and business consulting. With over 6 years of experience, Vidyesh has established a strong reputation for his proficiency in market estimations, supplier landscape analysis, and market share assessments for tailored research solution. Using his deep industry knowledge and analytical skills, he provides valuable insights and strategic recommendations, enabling clients to make informed decisions and navigate complex business landscapes.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




