Medical Affairs Outsourcing Market Size and Trends
Global medical affairs outsourcing market is estimated to be valued at USD 2.60 Bn in 2025 and is expected to reach USD 5.93 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 12.5% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Global medical affairs outsourcing market is witnessing growth due to rising R&D investment in drug development by pharmaceutical and biotechnology companies. Various advantages offered by medical affairs outsourcing such as reduced operational costs, access to experts and specialists, flexibility and scalability drives more companies to outsource their medical affairs requirements. Key market players are focusing on expanding service offerings such as medical information, medical communications and regulatory affair services to garner more share.
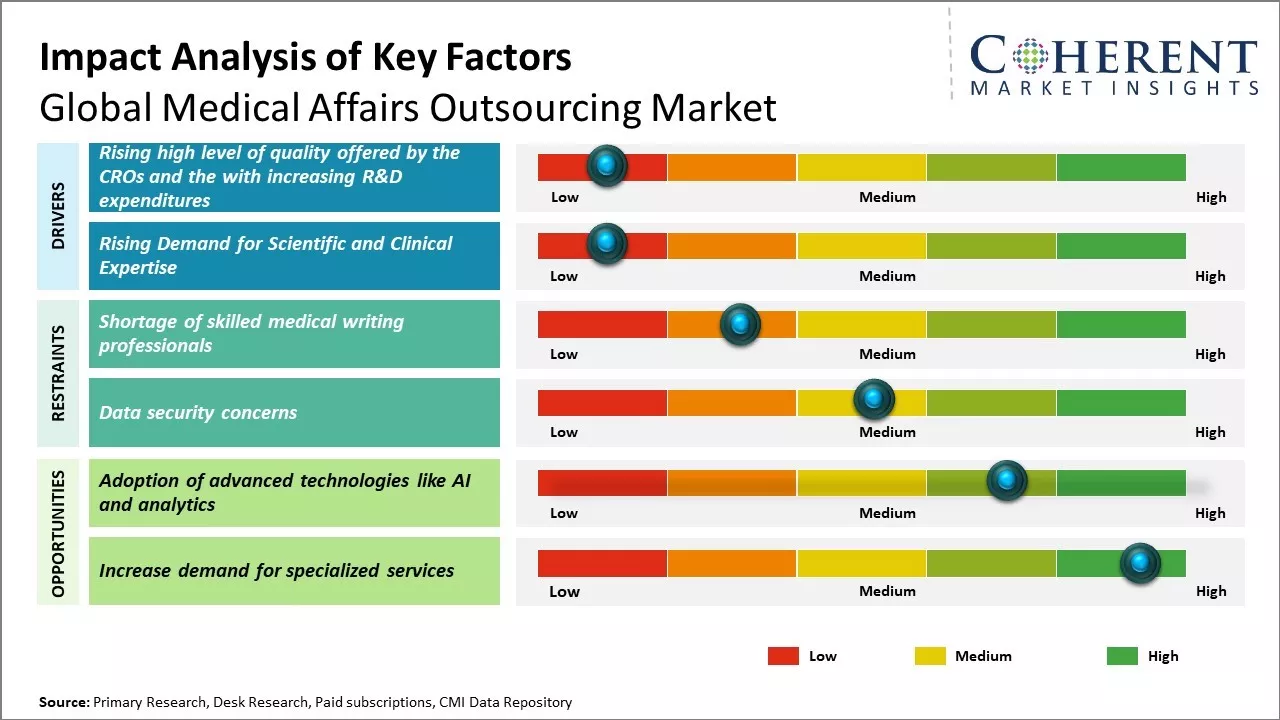
Rising high level of quality offered by the CROs with increasing R&D expenditures
Global medical affairs outsourcing market is witnessing significant growth due to increasing R&D expenditures coupled with rising clinical trial activity among sponsors. Due to growing complexity and cost of drug development, pharmaceutical and biotech companies are under immense pressure to launch new therapies faster and more efficiently. This has boosted demand for strategic outsourcing of medical affairs functions to contract research organizations (CROs) that can ensure end-to-end clinical trial support. CROs are now able to provide full spectrum medical expertise and services across the entire product lifecycle, from early discovery to commercialization and post marketing support. These have deep therapeutic experience and a global infrastructure to effectively handle worldwide trials. CROs also continuously invest in innovative technologies like artificial intelligence and analytics to improve protocol design, optimize site selection and management, enhance patient recruitment and retention. This high level of expertise, scale of operations and focus on digital solutions allow CROs to successfully deliver complex global programs within targeted timelines and budgets for their biopharma clients. Due to substantial investments in research and development and a robust pipeline of therapeutic developments, medical affairs outsourcing market will witness significant growth. For instance, according to the Pharmaceutical Industry and Global Health Facts and Figures 2021 Report, biopharmaceutical industry's annual research and development spending surpasses that of the aerospace and defense industries by 7.3 times, exceeds the chemicals industry by 6.5 times, and is 1.5 times greater than the software and computer services industry. This underscores the industry's strong commitment to advance medical innovations and underscores the anticipated expansion in medical affairs outsourcing services. In March 2021, according to an article published by Anju Life Sciences Software, enhanced data quality, improved safety decisions, lowered trial operation expenses, and accelerated study timelines boosts the success of early phase trials. This trend underscores the growing demand for outsourcing services provided by CROs.
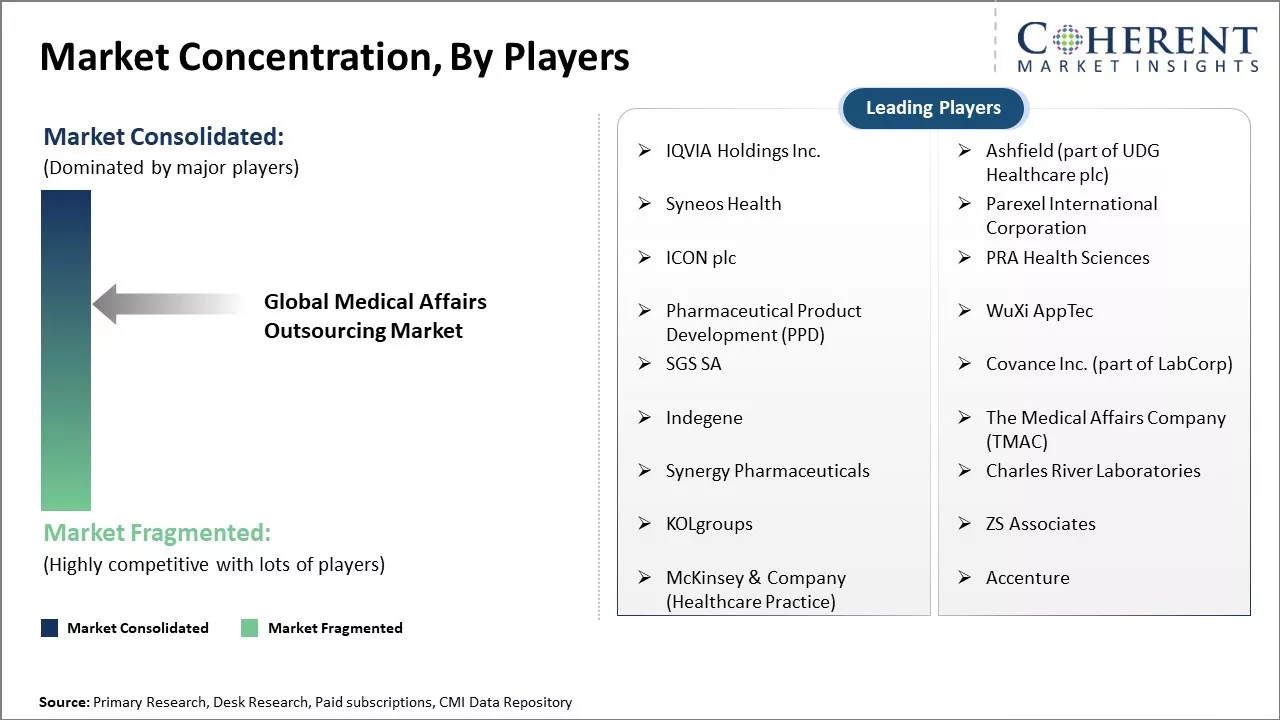
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Rising demand for scientific and clinical expertise
Global medical affairs outsourcing market growth is driven by growing needs of pharmaceutical and biotech companies for scientific and clinical expertise. With continuous evolution of science and advancement in research, there is constant development of new treatment options, drug delivery mechanisms, and diagnostic methods in the healthcare industry. However, most pharmaceutical and biotech companies find it challenging to hire and retain experienced medical affairs specialists with deep therapeutic area knowledge and clinical research skills in-house. Outsourcing medical affairs activities allows them to access a broader pool of scientific and therapeutic area experts on an ongoing basis without having to invest extensively in building internal medical capabilities. Medical affairs outsourcing vendors help life sciences organizations stay updated with the latest clinical evidence and treatment practices to effectively engage with healthcare professionals and gain their confidence in new products. This assumes more importance as clinical dossiers grow more comprehensive and regulators place greater emphasis on real-world effectiveness and safety of approved therapies. Access to specialized therapeutic knowledge and skillsets on demand through outsourcing has become critical for pharmaceutical companies as thse navigate an increasingly complex regulatory, clinical and marketplace environment.
Key Takeaways from Analyst:
Global medical affairs outsourcing market growth is driven by increased demand for outsourced medical affairs solutions. Pharmaceutical and biotech companies are under growing pressure to launch new therapies while reducing internal costs. This has pushed many sponsors to outsource non-core functions to specialized CROs. The market faces shortage of qualified medical science liaisons and therapeutic area experts. Medical affairs outsourcing helps sponsors plug this talent and expertise gap.
Asia Pacific is expected to be the fastest growing region for medical affairs outsourcing. Increasing clinical trial activity and drug launches in China, India and other Asian countries are driving regional sponsor companies to engage CROs for medical support. North America dominates the market as large sponsors in the U.S. rely on outsourcing for medical information and communication services.
Strict regulatory oversight across major markets can hamper the market growth. Sponsors may prefer to keep critical medical communication roles in-house to maintain full control over messaging. However, outsourcing partners are well equipped to navigate complex compliance guidelines. Furthermore, virtual technology solutions have enabled remote and flexible delivery models, helping overcome data security concerns.
Market Challenges: Shortage of skilled medical writing professionals
The shortage of skilled medical writers can hamper the global medical affairs outsourcing market growth. Medical writing plays a pivotal role in the medical affairs function by developing communication strategies, programs, content and other documentation to help life sciences companies adhere to compliance guidelines and improve patient outcomes. However, lack of qualified professionals who have an understanding of both medical and scientific concepts as well as experience in writing for external stakeholders such as other healthcare specialists, patients and regulatory agencies can hamper the market growth. This skill gap makes it difficult for medical affairs outsourcing companies to take up new projects and meet existing client demand in a timely manner. Due to rising complexity of therapeutics and regulations, medical documents need to be written and designed by experts who can translate complex outcomes and research into simple yet accurate language. However, many educational institutes are still catching up with industry needs and not producing enough medical writers with suitable training. This leads to high attrition rates at medical writing firms as existing talent is poached by larger players. Recruitment and training new hires can take from 6 months to 1 year depending on an individual's adaptability and competencies.
Market Opportunities: Adoption of advanced technologies like AI and analytics
Adoption of advanced technologies like AI and analytics provides a great opportunity for the global medical affairs outsourcing market growth. As the healthcare industry accumulates vast amounts of complex data from various sources like EMR systems, clinical trials, medical imaging, and others, AI and analytics can help unlock insights from this data. Pharmaceutical and medical device companies can leverage AI and analytics through outsourcing partners, to gain a deeper understanding of areas like patient outcomes, treatment effectiveness, adverse drug reactions and more. This clinical data analysis can help expedite drug discovery and development. AI tools that can sift through huge databases of research papers, clinical studies and unstructured text can also augment medical affairs functions. Applications of natural language processing and machine learning can accelerate literature monitoring and surveillance. It helps outsourcing companies to provide real-time, actionable insights to their life sciences clients. Advanced analytics further helps identify ideal patient populations for clinical trials and recruitment. It allows for better monitoring of trial endpoints and milestones.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
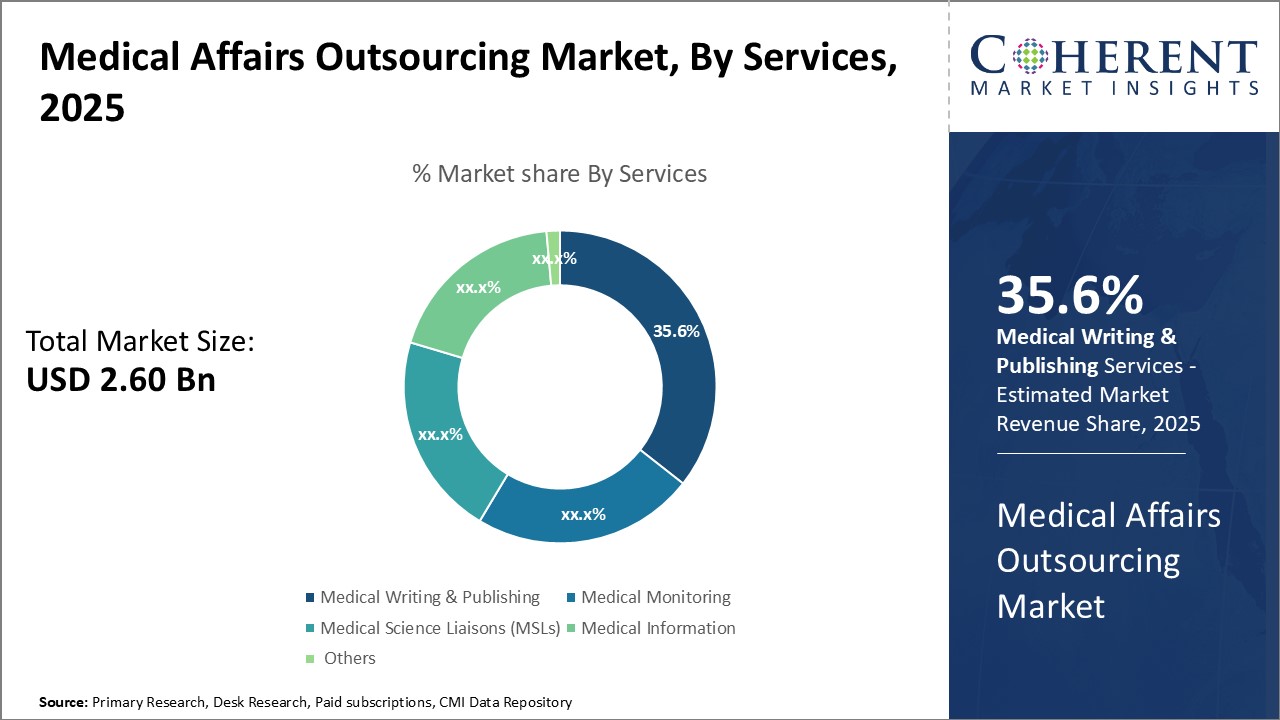
Insights, By Services: Medical writing & publishing segment dominates due to through stringent regulatory requirements
By services, medical writing & publishing segment is estimated to contribute highest market share of 35.6% in 2025 due to stringent regulatory norms. Pharmaceutical and biopharmaceutical companies are under immense pressure to publish high-quality scientific papers, clinical trial results, drug monographs, patient brochures, and other documentation to gain regulatory approvals. Medical writing agencies reduces the burden through specialized professionals trained in the nuances of scientific communication. These ensure documentation adheres to formatting guidelines of journals, regulatory style guides as well as plain language principles for patient-centric materials. Regulators worldwide have been increasing the quality standards and volume of information required at each stage of drug development. Firms now need robust integrated services that can plan, draft, review and publish materials according to global standards. Medical writers add value through their expertise in therapeutics areas, command over target languages and thorough understanding of submitting processes. These help expedite approvals through precisely addressing itemized queries and revision requests during review cycles. This allows pharmaceutical companies to launch new products faster. Furthermore, tight timelines for developing and publishing various submissions have boosted demand for scalable outsourced solutions. Medical writing agencies deploy sophisticated workflow management tools and capacity planning models to seamlessly handle high workloads. Their flexible resourcing also addresses fluctuations due to project-based nature of the work. The talent pool is carefully selected through rigorous certification programs to ensure consistency in quality and turnaround times. This has made medical writing & publishing a reliable and cost-effective option for companies to fulfil regulatory obligations without straining internal resources.
Insights, By Industry- Pharmaceutical industry dominates due to intensive R&D investments
By industry, pharmaceutical segment is estimated to contribute highest market share of 40.62% in 2025, owing to heavy investments in new drug development. Rising healthcare costs have prompted pharmaceutical giants to pursue more intensive research strategies targeting complex diseases. The expanding product pipelines require extensive operations to manage clinical trials, observational studies, registries and post-approval safety studies worldwide. Medical affairs outsourcing aids smooth coordination and reporting of such activities by providing on-demand scalable infrastructure and specialized talent. Outsourcing partners deploy advanced technologies like electronic data capture systems, interactive response systems and analytics platforms to efficiently design, setup and run diverse study models. Their embedded regulatory and therapeutic expertise helps navigate local ethical and legal guidelines. This allows seamless integration of global sites and participants, timely resolution of queries, and regional customization of informed consent procedures and documentation. Such tailored solutions have become critical for meeting enrollment goals and accelerated study timelines, especially for time-sensitive specialty drug programs.
Regional Insights
Need a Different Region or Segment? Download Free Sample
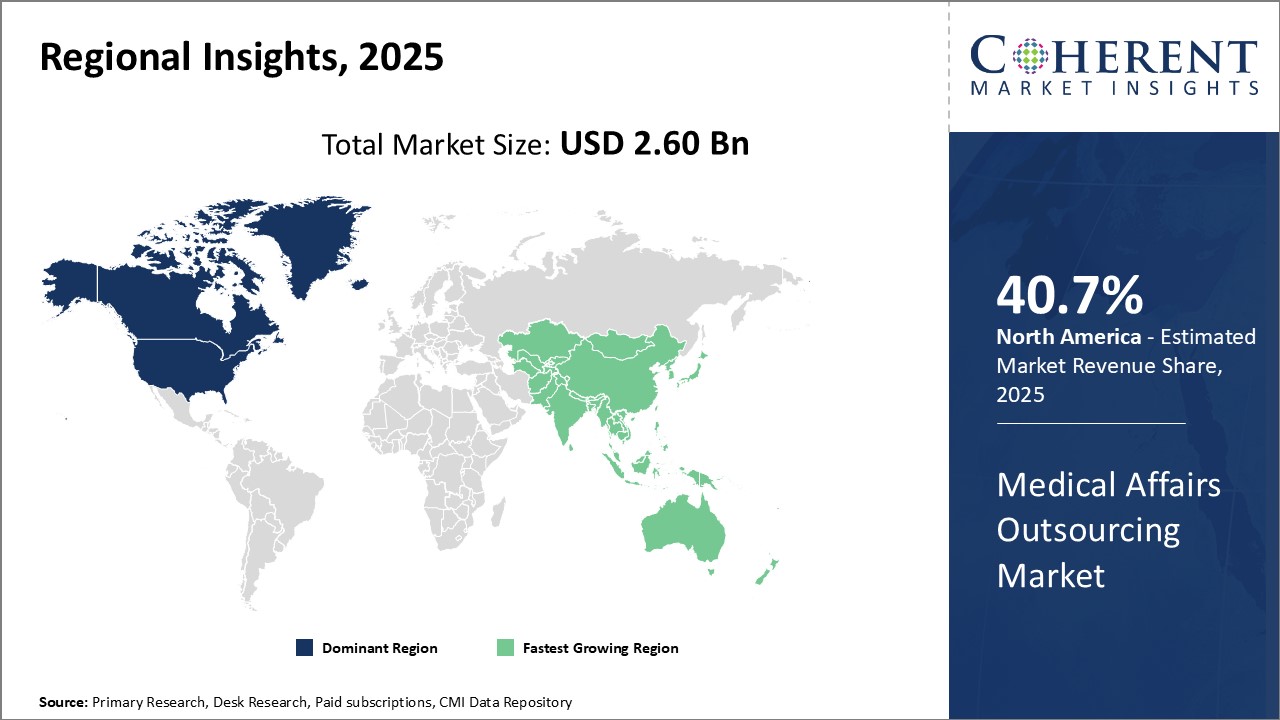
North America has been the dominant region in the global medical affairs outsourcing market with an estimated market share of 40.2 % in 2025. The strong presence of pharmaceutical and life sciences companies in the U.S. and Canada has boosted demand for outsourced medical support services. Leading pharmaceutical companies in the region are the early adopters of outsourcing non-core functions including medical affairs to focus on drug development. High costs of in-house medical teams and stringent regulatory compliance requirements have compelled more players to leverage experienced outsourcing partners. Key industry hubs such as Boston and New Jersey are major outsourcing destinations in North America, bolstered by the abundant talent pool available locally.
Asia Pacific has emerged as the fastest growing region for medical affairs outsourcing. Countries like India, China, South Korea and Singapore have witnessed massive expansion of their contract research and outsourcing industries. The availability of low-cost expertise, especially in functions like medical writing and scientific communication has attracted outsourcers to the Asia Pacific region. India has been proactively promoting medical research and services as a key strategic sector. Leading Indian CROs and KOL management companies have established themselves as efficient partners for post-marketing surveillance studies and other real-world evidence programs. Their experience in serving both international and domestic clients across various therapeutic domains has positioned the Asia Pacific region favorably. Growing pharmaceutical market and investments in healthcare research infrastructure in China and other ASEAN countries can drive the market growth.
Market Report Scope
Medical Affairs Outsourcing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.60 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.5% | 2032 Value Projection: | USD 5.93 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
IQVIA Holdings Inc., Ashfield (part of UDG Healthcare plc), Syneos Health, Parexel International Corporation, ICON plc, PRA Health Sciences, Pharmaceutical Product Development (PPD), WuXi AppTec, SGS SA, Covance Inc. (part of LabCorp), Indegene, The Medical Affairs Company (TMAC), Synergy Pharmaceuticals, Charles River Laboratories, KOLgroups, ZS Associates, McKinsey & Company (Healthcare Practice), Accenture |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Medical Affairs Outsourcing Industry News
- In March 2024, Celegence Holdings LLC, a life sciences company, invested in Soterius, Inc. through its Series A financing round. Soterius specializes in outsourced services, collaboration technologies, and data assets for drug safety and medical affairs. This partnership leverages the strengths of both organizations to enhance their reach and accelerate growth.
- In February 2024, Pfizer, a multinational pharmaceutical and biotechnology corporation, collaborated with NIPER Ahmedabad to foster and empower healthcare startups in India. This collaboration aims to nurture innovative ideas into market-ready solutions by supporting six selected innovators nationwide. These startups, chosen for their proof-of-concept aligned with the initiative's goals, will undergo a one-year accelerator program at NIPER Ahmedabad. The initiative, supported by the Department of Pharmaceuticals and Niti Aayog, is anchored by Social Alpha.
- In December 2021, RQM+, a global MedTech service provider, enhanced its regulatory expertise through the acquisition of AcKnowledge Regulatory Strategies. This addition integrates a highly skilled team into RQM+'s extensive network of FDA reviewers, engineers, scientists, and regulatory and quality specialists, bolstering the company's capabilities in FDA submission experience.
- In February 2021, ICON plc, a healthcare intelligence and clinical research organisation, completed the acquisition of PRA Health Sciences, Inc. for approximately USD 12 billion. This strategic move has significantly bolstered ICON's portfolio in medical affairs services, enhancing its service offerings in this critical sector.
*Definition: Global medical affairs outsourcing market provides various services to pharmaceutical, biotechnology and medical device companies to support their medical affairs and clinical development functions. These services include medical information, medical communications, medical monitoring, medical science liaisons, medical education, and consulting. Outsourcing medical affairs allows companies to access world-class scientific, regulatory and operational expertise to manage their medical programs in a more cost-effective way and help with pressing development timelines. It helps free up internal resources to focus on core competencies.
Market Segmentation
- Services Insights (Revenue, USD Bn, 2020 - 2032)
- Medical Writing & Publishing
- Medical Monitoring
- Medical Science Liaisons (MSLs)
- Medical Information
- Others
- Industry Insights (Revenue, USD Bn, 2020 - 2032)
- Pharmaceutical
- Biopharmaceutical
- Medical Devices
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- IQVIA Holdings Inc.
- Ashfield (part of UDG Healthcare plc)
- Syneos Health
- Parexel International Corporation
- ICON plc
- PRA Health Sciences
- Pharmaceutical Product Development (PPD)
- WuXi AppTec
- SGS SA
- Covance Inc. (part of LabCorp)
- Indegene
- The Medical Affairs Company (TMAC)
- Synergy Pharmaceuticals
- Charles River Laboratories
- KOLgroups
- ZS Associates
- McKinsey & Company (Healthcare Practice)
- Accenture
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
