Leber Congenital Amaurosis Market Size and Trends
The Global Leber Congenital Amaurosis Market is estimated to be valued at USD 1.29 Bn in 2025 and is expected to reach USD 1.78 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 4.7% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
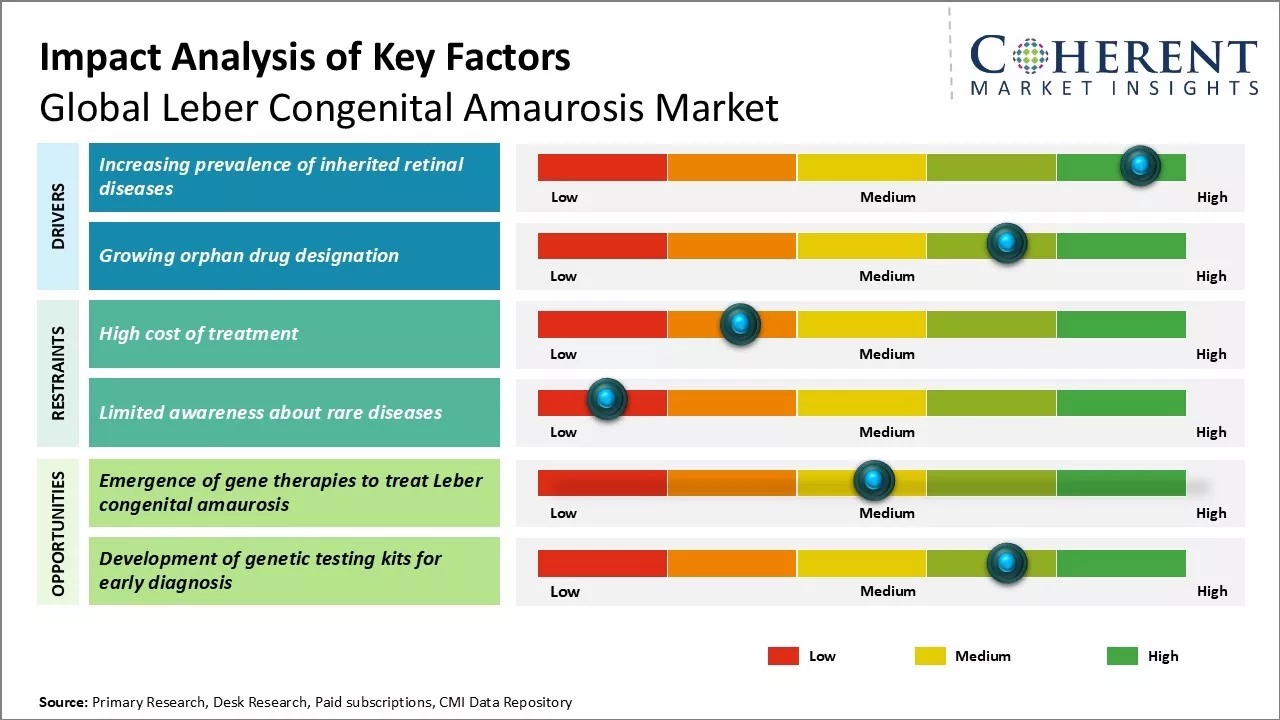
Increasing prevalence of inherited retinal diseases
The prevalence of inherited retinal diseases is increasing at an alarming rate across the globe. Leber congenital amaurosis, being one form of inherited retinal dystrophy, is also witnessing a spike in cases year-over-year. As per some estimations, around 2-3 out of every 100,000 live births are diagnosed with LCA. Given that the global birth rate averages around 140 million newborns annually, it means around 2,800 to 4,200 new LCA cases are emerging each year.
This upward trend can majorly be attributed to the advancing age of prospective parents. Medical science has enabled people to delay parenthood till late 30s or early 40s now unlike earlier times when most people had kids in their 20s. However, it is a well-established fact that the likelihood of inherited genetic disorders and mutations increase with increasing parental age. Studies have shown advanced paternal age to be a significant risk factor for several inherited eye diseases including LCA. Since more people are opting to become parents at an older age nowadays due to social and economic reasons, the chances of their offspring inheriting genetic retinal conditions like LCA grow correspondingly.
Another important reason for the rise in inherited retinal dystrophy prevalence is improving diagnosis. Availability of advanced genetic testing tools and techniques allows for faster and more definitive diagnosis of even rare retinal disorders. Previously, conditions like LCA which manifest from childhood often went undiagnosed for years due to the lack of expertise and diagnostic limitations. But now sophisticated genetic screening methods enable diagnosis even in newborn babies showing first signs of visual impairment. This has led to more accurate disease surveillance worldwide. Also, growing awareness about these genetic conditions thanks to patient advocacy groups is encouraging more families to go for diagnostic screening of family members, leading to identification of new cases.
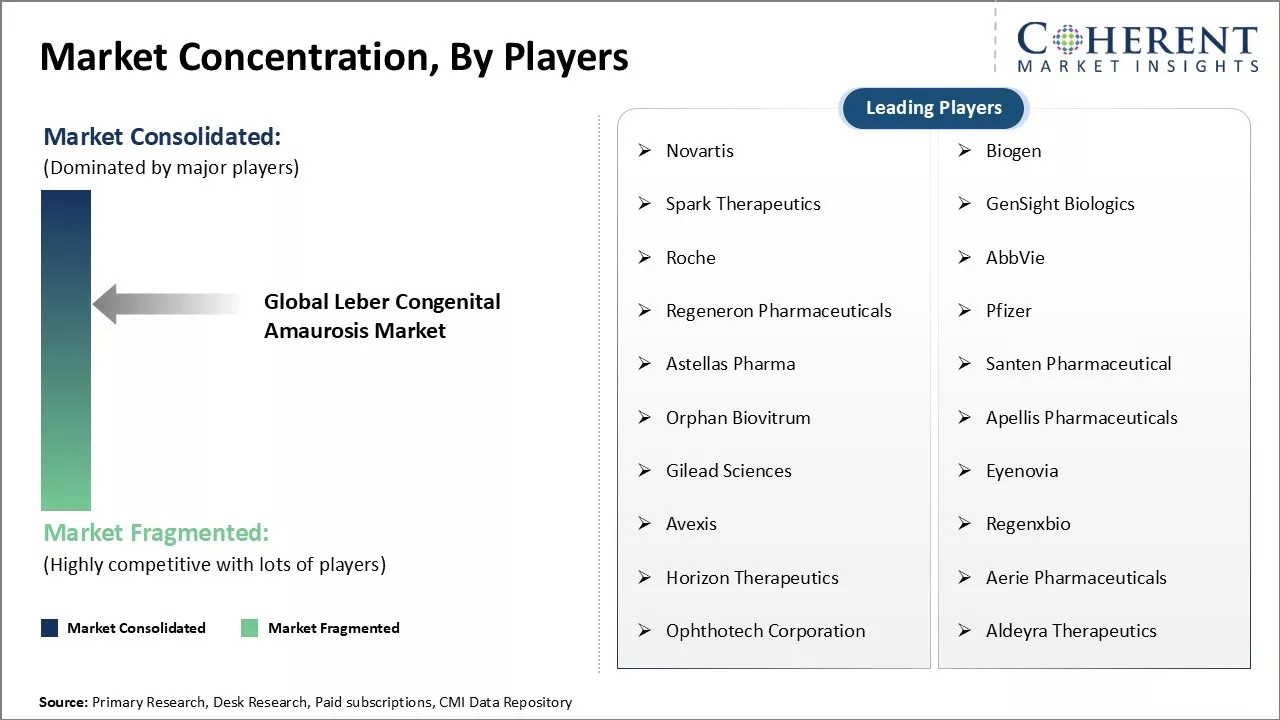
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Growing Orphan Drug Designation
Orphan drug status provides valuable incentives for pharmaceutical companies to invest more resources into research and development of therapies for rare diseases. Obtaining such designation opens up several benefits like clinical trial cost reimbursement, tax credits, exclusive rights over the designated drug for seven years post approval among others. More drug makers are thus actively pursuing orphan designation for their pipeline drugs focused on niche rare disease markets like LCA which impacts only a miniscule population.
Over the past decade, the regulatory landscape around orphan drugs has also evolved significantly. Both FDA and EMA have further strengthened the incentives and streamlined their approval pathways for orphan medicines to encourage innovation in areas of high unmet need. Pharma firms can now attain orphan drug designation even during early clinical trial stages based on plausible hypothesis of drug's clinical benefit. Additionally, regulators recognise the practical challenges of conducting traditional large scale efficacy studies for rare diseases and are more open to accept surrogate markers and real-world evidence instead.
A visible outcome of these policy developments is the rising number of orphan drug designations being awarded year-on-year. Many major players in ophthalmology too have obtained the coveted status. This demonstrates the commercial interest that rare retinal dystrophies like LCA represent nowadays. It becomes lucrative for drugmakers to invest in research and win designation so that they can enjoy market exclusivity should their candidate therapy succeeds. Consequently, the LCA pipeline is growing rapidly with multiple drug candidates at various stages of clinical evaluation having already secured orphan status from regulatory agencies. Their expected approvals in the coming years will significantly expand treatment options for LCA patients. Therefore, the proliferating orphan drug activity puts the global LCA market on a firm footing for steady growth.
Key Takeaways from Analyst:
The global Leber congenital amaurosis market is expected to witness significant growth over the forecast period. Increased awareness about the disease and various treatment options available is driving more patients to seek diagnosis and treatment. Government initiatives to support rare disease research is also fueling market growth.
However, high cost of treatment remains a major challenge for widespread adoption, especially in low and middle-income countries. Lack of experienced healthcare professionals to diagnose and manage the condition further restrains the market.
North America will continue dominating the global market supported by ongoing clinical trials and new product approvals. Favorable reimbursement policies are further boosting the regional market. Europe is also expected to see steady growth owing to growing geriatric population and increasing healthcare expenditure.
Asia Pacific offers huge untapped market potential and is poised to emerge as the fastest growing regional market in the coming years. This can be attributed to rising living standards, growing medical tourism, and initiatives to spread awareness about rare diseases.
New product launches with better efficacy and safety are paving the way for incremental growth opportunities. Pipeline candidates bode well for future market expansion. Collaborations between key industry stakeholders to make affordable treatment available worldwide present lucrative opportunities.
Market Challenges: High cost of treatment
One of the major challenges for the global Leber congenital amaurosis market is the high cost of treatment. Leber congenital amaurosis is a rare genetic disorder that causes severe visual impairment from birth. There are currently no approved drug therapies for treating this condition, and the available treatments like gene therapies come with exorbitant price tags. For example, voretigene neparvovec, the first FDA approved gene therapy drug for LCA, has a list price of $850,000 per treatment. Such astronomical prices put an enormous financial burden on patients, healthcare systems, and governments. Many patients will not be able to afford these costly treatments without proper insurance coverage or financial assistance. The high costs can also limit access to treatment and negatively impact the market potential of new therapies. Companies developing gene and other advanced therapies will need to balance innovation with affordability to make treatments accessible to more patients on a global scale.
Market Opportunities: Emergence of gene therapies to treat Leber congenital amaurosis
The emergence of gene therapies offers a significant opportunity for the global Leber congenital amaurosis market. Since LCA is primarily caused by mutations in specific genes, gene therapy is considered a highly viable option with potential for one-time curative treatment. Several drug makers are actively developing gene therapies with promising outcomes in clinical trials. Voretigene neparvovec became the first approved gene therapy in 2021. Other therapies in late-stage trials include AVROBIO's AVR-RD-01, MeiraGTx’s AAV-CNGB3, and AGTC's XLRP Therapy. The approval and success of initial gene therapies is expected to validate this approach and attract more investments into the space. It could also pave the way for the development of additional gene therapies targeting different genetic causes of LCA. With continued innovation, gene therapies may become an important standard of care for managing Leber congenital amaurosis in the future.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
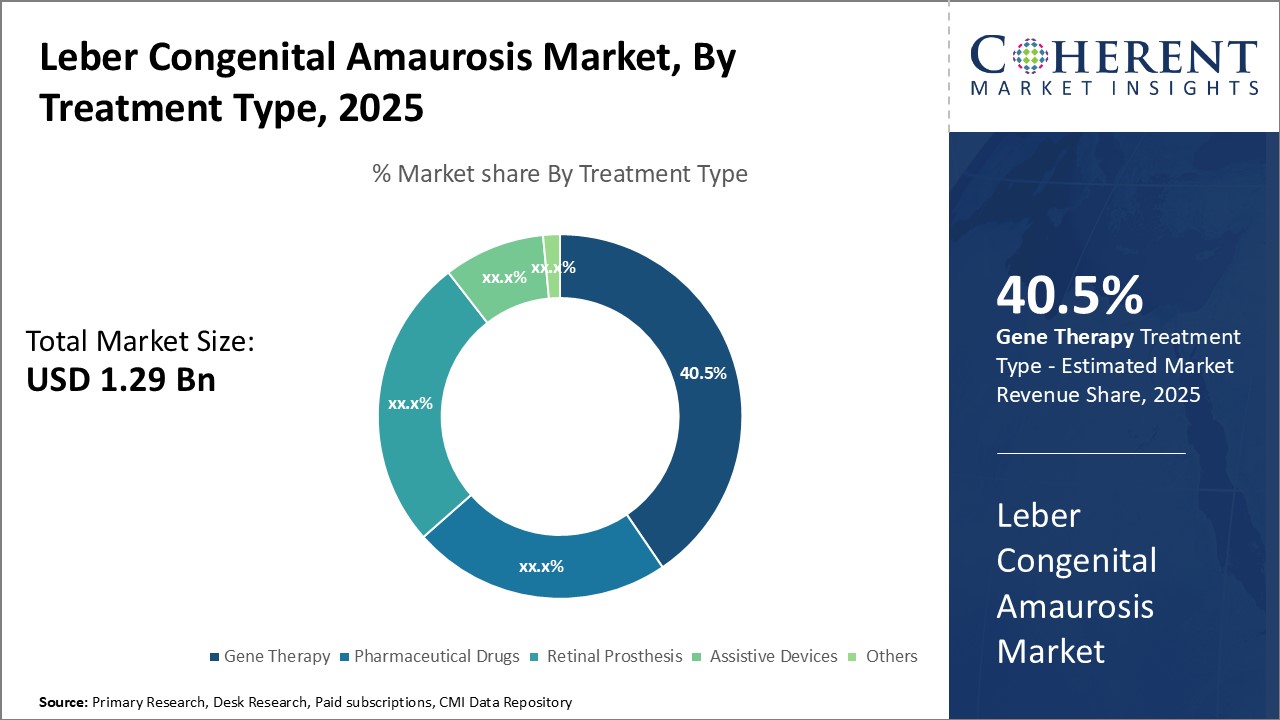
By Treatment Type - Gene Therapy: fueling innovation & hope
In terms of Treatment Type, the Gene Therapy segment is estimated to hold the highest share of 40.5% in 2025 owing to continuous advancements in vector technology and delivery methods. Gene therapy aims to correct the genetic defect causing Leber Congenital Amaurosis by introducing a functional copy of the defective gene. Compared to other options, gene therapy offers a one-time treatment with long-lasting effects. Significant research is being focused on improving the safety and efficiency of viral vectors. Innovative delivery techniques through suprachoroidal and subretinal injections are enhancing targeting of retinal cells. Promising clinical trials of therapies targeting RPE65 and other genes have generated momentum. The success of voretigene neparvovec in treating RPE65-mediated LCA has reinforced the potential of gene therapy. If regulatory approval is achieved, several gene therapy candidates currently in late-stage testing may enter the market. This will strengthen gene therapy's position as the preferred treatment approach.
By Target Gene - RPE65: driving hope through genetic targeting
In terms of Target Gene, the RPE65 segment is estimated to hold the highest share of 35.5% in 2025 owing to advances in RPE65 gene therapy. RPE65 mutations account for a substantial percentage of total LCA cases. Therapies targeting RPE65 have shown clinically meaningful results in restoring vision. Luxturna became the first FDA-approved gene therapy for an inherited retinal disease by targeting RPE65. It demonstrated long-lasting benefits through a single administration. This validated RPE65 as a viable gene target. Emerging therapies like ADVM-022 also aim to utilize the AAV vector platform to deliver the RPE65 gene. Extensive research pathways are optimizing vector engineering and refining targeting of retinal cells. Given success so far, RPE65 is likely to remain the frontrunner gene target as more products enter clinical evaluation and commercialization. This would solidify its share of the LCA treatment market.
By End-User - Hospitals: fostering integrated care
In terms of End-User, the Hospitals segment is estimated to hold the highest share of 42.5% in 2025 owing to their ability to provide comprehensive care. As the condition requires multidisciplinary management and coordination between specialists, hospitals are well-equipped to handle the diverse needs of LCA patients. They offer on-site retinal specialists, genetic testing facilities, gene therapy administration capabilities, low vision devices and vision rehabilitation programs under one roof. This integrated healthcare delivery model fosters effective long-term monitoring and follow-ups. As novel therapies emerge, hospitals will play a key role in large-scale clinical trials as treatment centers. With skilled expertise across departments, hospitals also guide patients through complicated procedures and stay updated on the rapidly evolving treatment landscape. Going forward, they are well-positioned to adopt complex formulations like gene therapies and lead in areas like data registries for treated patients. This will enable hospitals to uphold their position as the dominant healthcare setting for LCA patients.
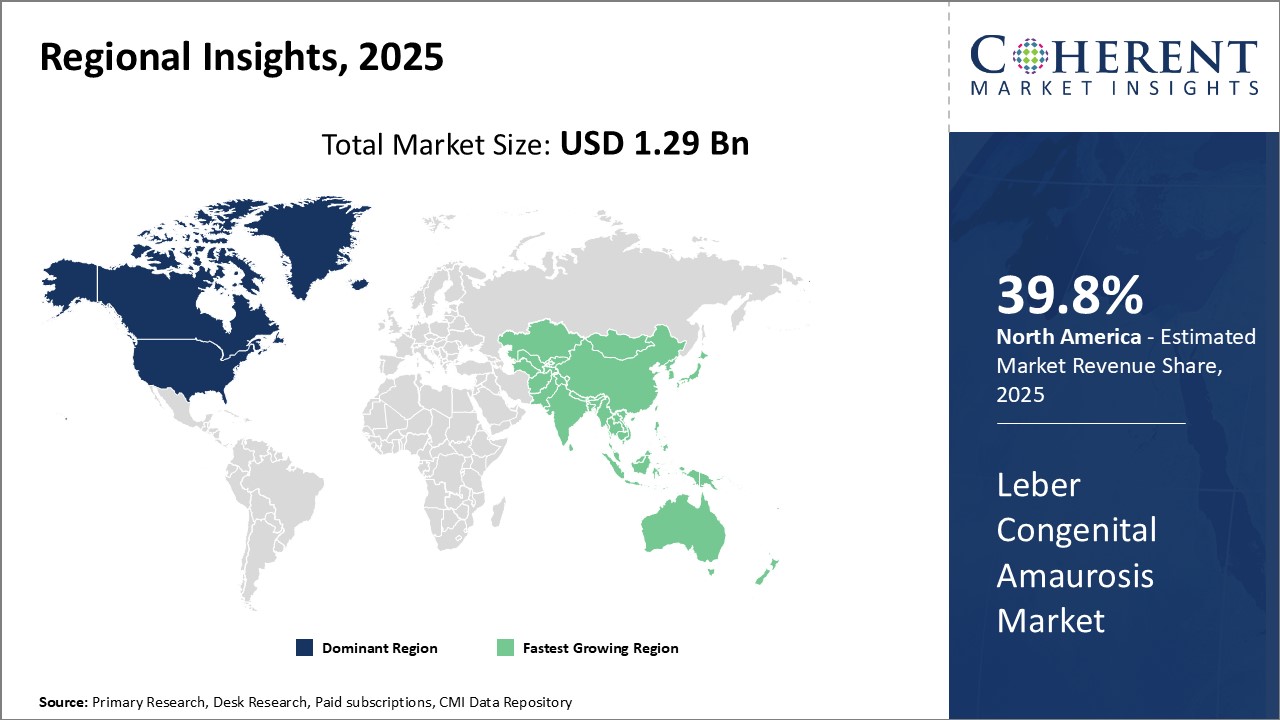
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America has established itself as the dominant region in the global Leber congenital amaurosis market with an estimated share of 39.8% in 2025. Majority of key market players are headquartered in countries like the U.S. and Canada. This has given North American companies an edge in terms of industry presence and market reach. The region is home to some of the largest pharmaceutical companies with significant investments in R&D. As a result, many pathbreaking gene therapies and drugs for treating LCA have been introduced first in the North American market.
Furthermore, the developed healthcare infrastructure and high healthcare expenditure in the region have ensured greater patient access to these high-priced therapies. This has goaded companies to focus more on this region for commercialization of new treatments. Export of LCA therapies from North America has also been substantial to other developed markets. Major patent expirations over the coming years could pose a challenge, but North American firms still hold an edge with slew of new pipeline drugs. Overall, the strong innovation ecosystem and robust commercialization environment have positioned North America as the dominant force in the global LCA market.
Asia Pacific region, especially China and India, is projected to witness the fastest growth over the forecast period. Growing medical needs of the expanding patient population coupled with rising healthcare investments present lucrative opportunities. This has spurred several multinational companies to focus on the Asia Pacific region through partnerships, joint ventures, and investments.
Moreover, the lowering of import duties and development of local manufacturing capacities are expected to improve access and bring down prices of drugs in the long run. China especially has been proactively working towards building an efficient regulatory framework to attract investments in advanced therapies. With its large untapped market potential, Asia Pacific's LCA market seems set to grow at an exponential rate compared to matured Western markets. Collaborations between global innovators and local Asian companies will likely accelerate product adoption across the region.
Market Report Scope
Leber Congenital Amaurosis Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.29 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.7% | 2032 Value Projection: | USD 1.78 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Novartis, Biogen, Spark Therapeutics, GenSight Biologics, Roche, AbbVie, Regeneron Pharmaceuticals, Pfizer, Astellas Pharma, Santen Pharmaceutical, Orphan Biovitrum, Apellis Pharmaceuticals, Gilead Sciences, Eyenovia, Avexis, Regenxbio, Horizon Therapeutics, Aerie Pharmaceuticals, Ophthotech Corporation, and Aldeyra Therapeutics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Leber Congenital Amaurosis Industry News
- In April 2023, Researchers from the National Eye Institute found that Reserpine, an FDA-approved drug originally used for high blood pressure, shows potential for treating Leber congenital amaurosis type 10 (LCA10). In laboratory models, Reserpine helped preserve light-sensitive photoreceptors affected by LCA10, a genetic disorder caused by CEP290 mutations.
- In November 2022, Editas Medicine presented clinical data from the Phase 1/2 BRILLIANCE trial of EDIT-101, a CRISPR/Cas9-based therapy for Leber congenital amaurosis 10 (LCA10), a rare retinal disorder. The trial showed that three of the 14 patients treated experienced significant improvements in best corrected visual acuity (BCVA) and other visual function measures. EDIT-101 targets the IVS26 CEP290 mutation, affecting around 1,500 patients in the U.S., for which there is currently no effective treatment.
*Definition: The Global Leber Congenital Amaurosis Market focuses on the treatment of the rare genetic disorder called Leber congenital amaurosis (LCA), which causes early visions loss or blindness from birth. The market involves research, development, and commercialization of therapies like gene therapy, vision testing, and counseling to manage the disease symptoms. It analyzes the regulatory guidelines and reimbursement scenario supporting the availability of LCA treatments.
Market Segmentation
- By Treatment Type Insights (Revenue, USD Bn, 2020 - 2032)
-
- Gene Therapy
- Pharmaceutical Drugs
- Retinal Prosthesis
- Assistive Devices
- Others
- By Target Gene Insights (Revenue, USD Bn, 2020 - 2032)
- RPE65
- GUCY2D
- AIPL1
- RPGRIP1
- CEP290
- Other Genes
- By End-user Insights (Revenue, USD Bn, 2020 - 2032)
- Hospitals
- Specialized Eye Clinics
- Ophthalmology Research Centers
- Home Care Settings
- Others
- By Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Novartis
- Biogen
- Spark Therapeutics
- GenSight Biologics
- Roche
- AbbVie
- Regeneron Pharmaceuticals
- Pfizer
- Astellas Pharma
- Santen Pharmaceutical
- Orphan Biovitrum
- Apellis Pharmaceuticals
- Gilead Sciences
- Eyenovia
- Avexis
- Regenxbio
- Horizon Therapeutics
- Aerie Pharmaceuticals
- Ophthotech Corporation
- Aldeyra Therapeutics
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
