Global Leadless Pacemakers Market Size and Share Analysis
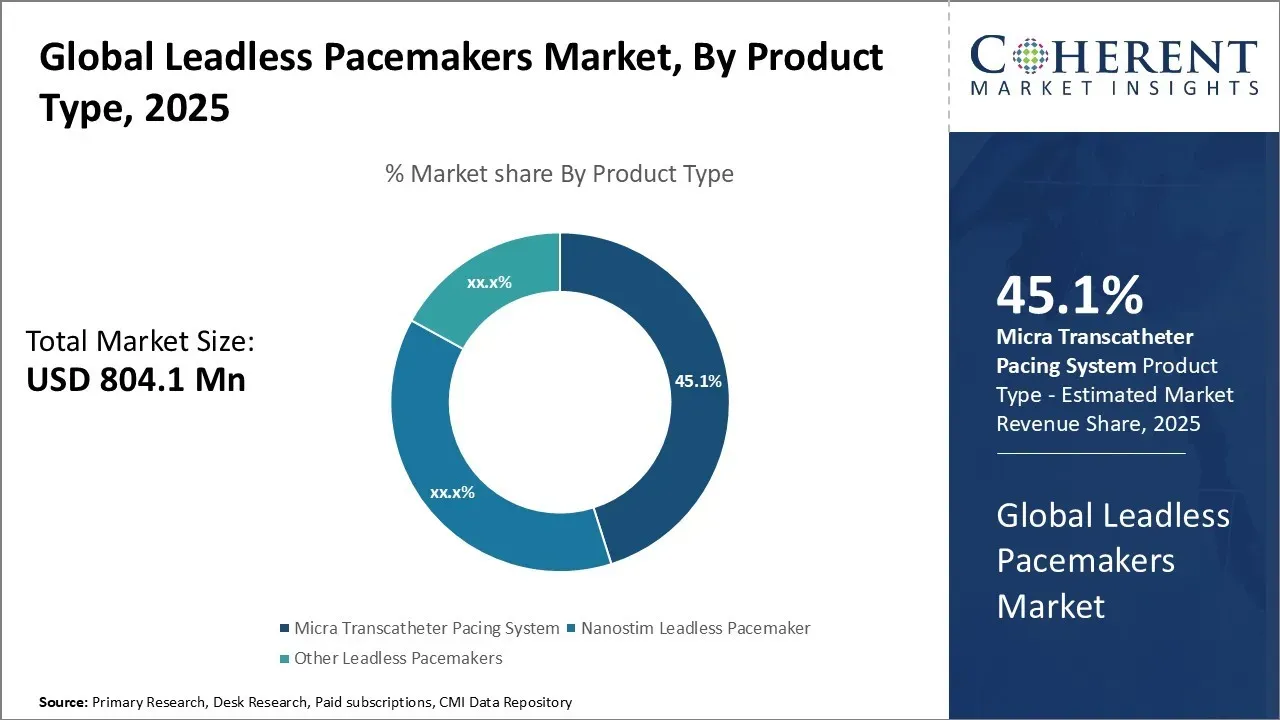
The global leadless pacemakers market is estimated to be valued at USD 804.1 Mn in 2025 and is expected to reach USD 1,965.2 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 13.6% from 2025 to 2032.
To learn more about this report, Download Free Sample
Key Takeaways
- By Product Type, the Micra Transcatheter Pacing System Segment is expected to contribute the highest share of the market with 45. 1%in 2025.
- By Type, Single Chamber Segment is expected to contribute the highest share of the market with 51.2% in 2025.
- By End User, Hospitals Segment is expected to contribute the highest share of the market with 36.2% in 2025.
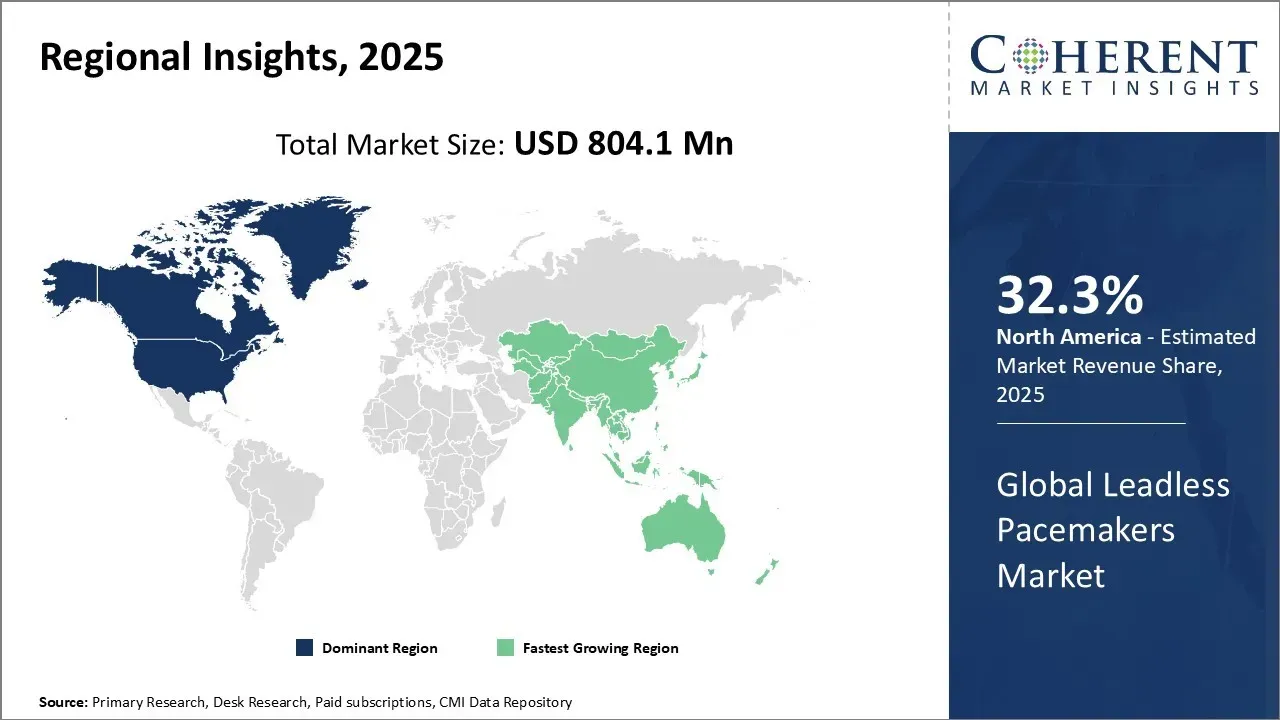
- North America is expected to top the global market with 32.3% share in 2025.
Market Overview
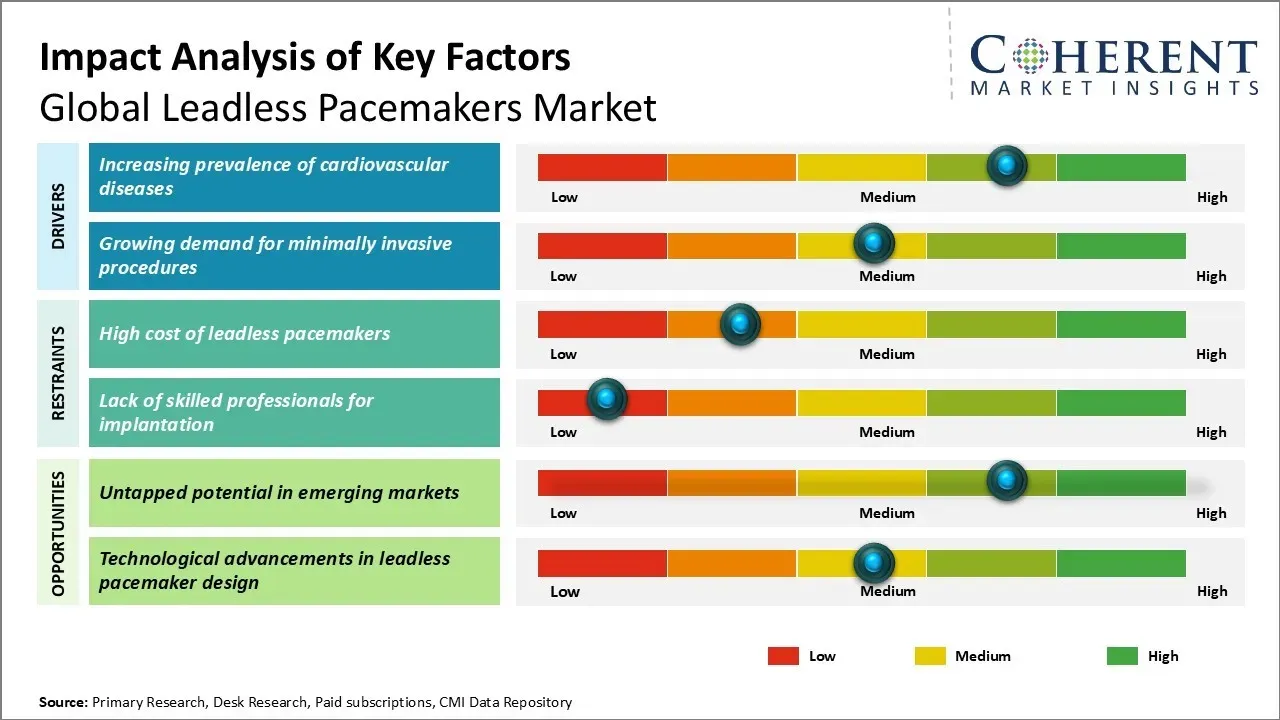
The leadless pacemakers market is showcasing significant growth due to the increasing prevalence of cardiovascular diseases and the rising geriatric population globally. The emerging technologies and innovations in leadless pacemakers are likely to provide opportunities for manufacturers. Miniaturization and battery longevity are some developments expected to positively impact the market. However, stringent regulatory approvals and high cost of these devices may hamper market growth to some extent during the analysis period.
In April 2025, researchers at Northwestern University unveiled the world’s smallest leadless pacemaker, measuring just 1.8 mm in width and 3.5 mm in length—smaller than a grain of rice. This innovative device is designed for temporary use, particularly in newborns with congenital heart defects.
Current Events and its Impact on bacon market
|
Current Events |
Description and its impact |
|
Miniaturization and Pediatric Advances Progress with Bioabsorbable Pacemaker Breakthrough |
|
|
Launch of Dual-Chamber Leadless Pacemakers by Abbott |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Players, Key Development, and Competitive Intelligence
To learn more about this report, Download Free Sample
Market Driver - Increasing prevalence of cardiovascular diseases
The rising number of cardiovascular diseases across the globe has led to the increasing adoption of leadless pacemakers. Cardiovascular diseases are among the major causes of mortality and morbidity worldwide. The major risk factors associated with cardiovascular diseases such as obesity, physical inactivity, tobacco use, and harmful use of alcohol are on the rise.
Developing countries are experiencing a huge economic and health burden due to the increasing incidence of cardiovascular diseases as people adopt sedentary lifestyles and harmful habits. Leadless pacemakers are being widely used for patients suffering from bradycardia which is a slow or irregular heartbeat and often requires lifelong treatment.
The small and leadless design of these pacemakers allows them to be implanted using minimally invasive method, reducing complications for patients. According to reports published by the National Library of Medicine in June 2023, the prevalence of arrhythmias in the general population was estimated to be between 1.5% and 5%. Among these cardiac irregularities, atrial fibrillation (AF) has emerged as the most common subtype.
Market Opportunity - Untapped potential in emerging markets
There is a huge untapped growth opportunity for leadless pacemakers in emerging market economies. While developed markets in North America and Europe currently dominate the usage of leadless pacemakers, developing countries in Asia Pacific, Latin America, the Middle East, and Africa account for a major share of the total patient pool requiring pacemaker therapy.
However, penetration of leadless pacemaker technology remains very low in emerging markets due to inadequate healthcare infrastructure and the high device costs mentioned earlier. As device prices gradually fall through continuing innovations and as emerging market healthcare systems expand their coverage for these novel devices, there will be immense scope for greater adoption.
Leading providers would do well to devise strategies to tap into these emerging geographies where the demand for pacemaker therapies is substantial but currently unmet due to access barriers.
Leadless Pacemakers Market Insights, By Product Type
In terms of product type, the micra transcatheter pacing system segment is expected to contribute the highest share of the market with 45.1% in 2025 owing to its advanced technological design and miniaturized size. Being the world's smallest pacemaker, micra is entirely implantable via catheter and avoids the need for surgical incision. Its miniature electrode fits comfortably inside the heart chamber ensuring minimal complications.
The device's sophisticated sensors and circuitry allow for precise monitoring and pacing functions in a package that is only 20mm long and 5mm wide. This has opened up leadless pacing to broader base of patients including those deemed too high-risk for conventional devices. The design breakthroughs have also enhanced procedural safety and reduced operator learning curve.
Leadless Pacemakers Market Insights, By Type
In terms of type, the single chamber segment is expected to contribute the highest share of the market with 51.2% in 2025 owing to better anatomical fit for majority of patients. Most bradycardia cases are managed effectively with single site pacing within the right ventricle or right atrium. The single chamber leadless pacemakers are tailored for these common conditions limiting positioning to a single heart site.
Their simpler design and smaller size prevent potential complications that may arise with multi-site pacing. The avoidance of scheduling additional leads and electrodes streamlines device implantation as well. Moreover, single chamber option presents a lower upfront cost and longer-term cost savings over dual chamber alternatives. These factors have enabled this segment to command the largest consumer base looking for technically feasible and affordable pacing solution.
Leadless Pacemakers Market Insights, By End User
In terms of end user, the hospitals segment is expected to contribute the highest share of the leadless pacemaker’s market with 36.2% in 2025 owing to accessibility to intensive care infrastructure and expertise in complex implantation procedures. Leadless pacemaker placement requires a sterile operating room setup, state-of-art fluoroscopic imaging, and skilled hands experienced in catheter manipulation and navigation within the heart.
Most hospitals are equipped to offer such facilities and have electrophysiologists or cardiac surgeons well-trained in leadless pacing techniques on their rolls. The in-house expertise helps tackle any unexpected challenges during implantation safely without delay. It also enables effective perioperative care and monitoring. Moreover, larger volume of such procedures performed at hospitals help clinicians maintain their critical skills.
Regional Insights
To learn more about this report, Download Free Sample
North America Leadless Pacemakers Market Trends
In North America, the dominance in the leadless pacemaker’s market with a projected share of 32.3% in 2025 can be attributed to factors such as the growing disease prevalence, supportive healthcare infrastructure and policies, and the presence of major players. The region has witnessed rapid technological advancement and adoption.
Asia Pacific Leadless Pacemakers Market Trends
Meanwhile, the Asia Pacific region is expected to exhibit the fastest growth with a projected share of 25.4% in 2025, driven by expanding medical tourism, growing healthcare spending, and increasing focus on preventive healthcare. Countries aim to bridge the infrastructure gap with the west.
Leadless Pacemakers Market Dominating Countries
U.S. Leadless Pacemakers Market Trends
The U.S. leadless pacemakers’ market is poised for significant growth, driven by rapid technological advancements, rising incidence of cardiovascular diseases, and favorable reimbursement policies. According to Centers for Disease Control and Prevention, for instance, in October 2024, heart disease became the leading cause of death in the U.S., accounting for 702,880 deaths in 2022. Coronary artery disease (CAD) is the most common type, affecting 5% of adults and causing 371,506 deaths that year. Heart attacks occur every 40 seconds, with 805,000 cases annually. Heart disease costs reached USD 252.2 billion between 2019 and 2020.
U.K. Leadless Pacemakers Market Trends
The U.K. is a significant player in the Europe leadless pacemakers’ market, fueled by a growing geriatric population and a strong focus on patient safety. The demand for leadless pacemakers is rising due to their advantages over traditional devices, particularly for patients at higher risk of complications.
India Leadless Pacemakers Market Trends
India leadless pacemakers’ market is experiencing rapid growth, driven by factors such as the rising prevalence of cardiovascular disorders, improved healthcare infrastructure, and increasing awareness of advanced cardiac care options. The preference for leadless pacemakers in India stems from their benefits over traditional pacemakers, including lower infection risks, fewer lead-related complications, and enhanced patient comfort. This trend is particularly notable among patients seeking minimally invasive treatments.
Germany Leadless Pacemakers Market Trends
Germany represents a significant revenue contributor to the Europe leadless pacemakers’ market, boasting the highest pacemaker implant rates in the region. This reflects a strong demand for advanced cardiac devices, including leadless pacemakers. The country’s well-established healthcare infrastructure, highly skilled medical professionals, and supportive regulatory framework drive its market leadership.
Market Report Scope
Leadless Pacemakers Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 804.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.6% | 2032 Value Projection: | USD 1,965.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
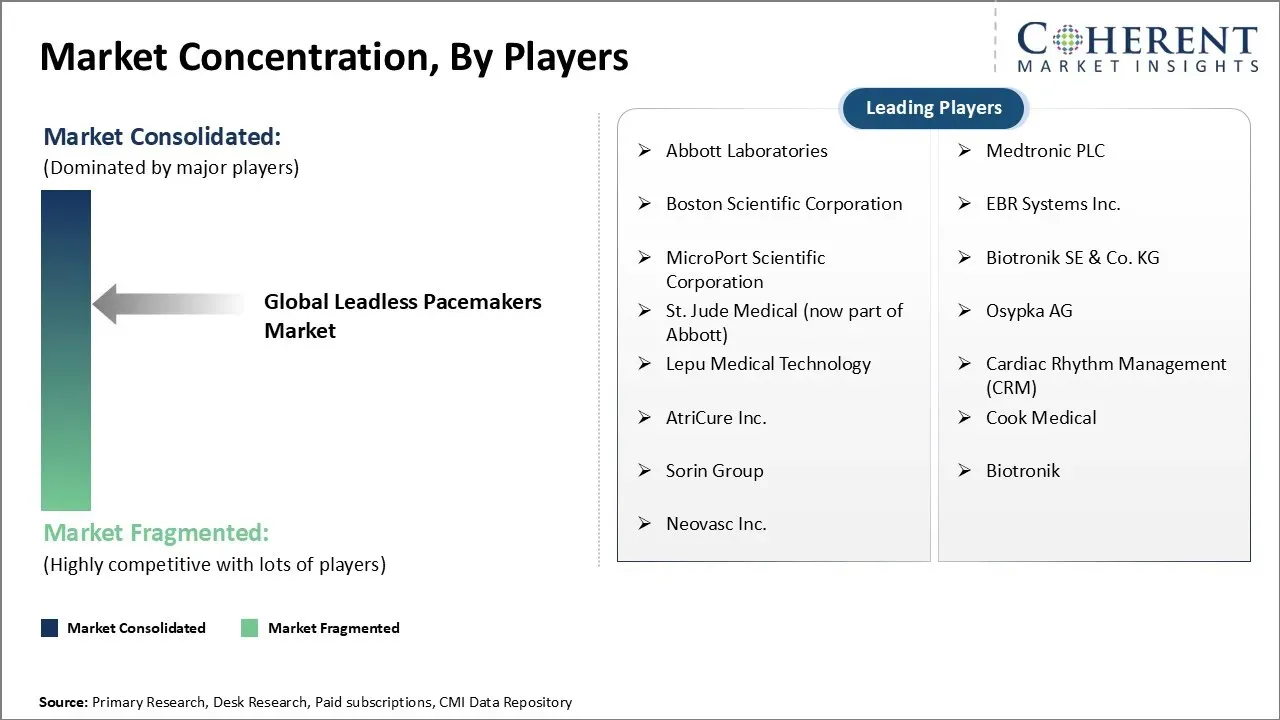
Abbott Laboratories, Medtronic PLC, Boston Scientific Corporation, EBR Systems Inc., MicroPort Scientific Corporation, Biotronik SE & Co. KG, St. Jude Medical (now part of Abbott), Osypka AG, Lepu Medical Technology, Cardiac Rhythm Management (CRM), AtriCure Inc., Cook Medical, Sorin Group, Biotronik, and Neovasc Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Key Developments
- In November 2024, Abbott launched the AVEIR VR (Ventricular Rate) leadless pacemaker in India, following approval by the Central Drugs Standard Control Organization (CDSCO) and the U.S. Food and Drug Administration (FDA). This advanced device, designed for patients with slow heart rhythms, is implanted directly into the heart’s right ventricle via a minimally invasive catheter-based procedure, eliminating the need for chest incisions or leads.
- In May 2024, Medtronic, a pioneer in healthcare technology, advanced pacemaker innovation with the U.S. FDA approval of its Micra AV2 and Micra VR2. These miniaturized, leadless devices offer extended battery life and easier programming, broadening access to this therapy.
- In January 2024, Medtronic Plc., a healthcare technology company, received the Conformité Européenne (CE) Mark for its Micra AV2 and Micra VR2, the next-generation miniature leadless pacemakers, following U.S. FDA approval in 2023. These devices, the smallest pacemakers globally, offer 40% longer battery life (16–17 years) and easier programming, with over 80% of patients needing only one device for life.
Analyst Opinion
- The global leadless pacemakers’ market has significant growth potential over the next decade. These novel pacemaker devices have emerged as an innovative alternative to traditional transvenous pacemakers for treating Bradyarrhythmia’s. Their miniaturized design provides several clinical advantages like easier implantation through femoral or brachial access with reduced risks of lead complications.
- This has boosted patient adoption and acceptance of the technology. The market is expected to witness strong demand globally, driven by the growing geriatric population base that is more prone to conduction disorders. Regions with aging demographics like North America, Western Europe will likely contribute most to revenue generation.
- High costs of leadless pacemakers may restrict their widespread use, especially in developing Asian, Latin American, and Middle Eastern markets in the short-term. Furthermore, clinical evidence is still needed on the longevity and safety profile of these devices for long-term use. Stringent regulatory approval processes also pose a challenge for market entry of new players.
- Opportunities lie in developing updated dual-chamber versions and remote monitoring capabilities. Strategic partnerships between device manufacturers and technology companies will help expand indications through ongoing trials.
Market Segmentation
- Product Type Insights (Revenue, USD Mn, 2020 - 2032)
-
- Micra Transcatheter Pacing System
- Nanostim Leadless Pacemaker
- Other Leadless Pacemakers
- Type Insights (Revenue, USD Mn, 2020 - 2032)
- Single Chamber
- Dual Chamber
- End User Insights (Revenue, USD Mn, 2020 - 2032)
- Hospitals
- Ambulatory Surgical Centers
- long-term care facilities
- Others
- Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Abbott Laboratories
- Medtronic PLC
- Boston Scientific Corporation
- EBR Systems Inc.
- MicroPort Scientific Corporation
- Biotronik SE & Co. KG
- Jude Medical (now part of Abbott)
- Osypka AG
- Lepu Medical Technology
- Cardiac Rhythm Management (CRM)
- AtriCure Inc.
- Cook Medical
- Sorin Group
- Biotronik
- Neovasc Inc.
Sources
Primary Research Interviews
- Cardiologists and Electrophysiologists
- Medical Device Manufacturers and R&D Personnel
- Hospital Procurement Managers and Healthcare Administrators
- Regulatory Affairs Specialists
- Others
Databases
- FDA 510 (k) Premarket Notification Database
- ClinicalTrials.gov
- Medical Device Database (GUDID)
- Healthcare Cost and Utilization Project (HCUP)
- Others
Magazines
- Medical Device and Diagnostic Industry (MDDI)
- Cardiovascular Business Magazine
- Medical Design & Outsourcing
- EP Lab Digest
- Others
Journals
- Journal of the American College of Cardiology (JACC)
- Heart Rhythm Journal
- Europace Journal
- Others
Newspapers
- The Wall Street Journal (Healthcare Section)
- Financial Times (Medical Technology Coverage)
- Reuters Health News
- Bloomberg Healthcare
- Others
Associations
- Heart Rhythm Society (HRS)
- American College of Cardiology (ACC)
- European Society of Cardiology (ESC)
- AdvaMed (Advanced Medical Technology Association)
- Others
Public Domain Sources
- Centers for Medicare & Medicaid Services (CMS)
- World Health Organization (WHO) Reports
- National Heart, Lung, and Blood Institute (NHLBI)
- European Medicines Agency (EMA) Publications
- Others
Proprietary Elements
- CMI Data Analytics Tool
- Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients