Inhaled Nitric Oxide Market Size and Trends
The Inhaled Nitric Oxide Market is estimated to be valued at USD 1.31 Bn in 2025 and is expected to reach USD 2.21 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.7% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The non-invasive nature of inhaled nitric oxide and its selective vasodilating property helps in improving oxygenation in newborn babies suffering from hypoxic respiratory failure. This makes inhaled nitric oxide an attractive therapeutic option over other conventional vasodilators. Furthermore, rising incidences of pre-term births worldwide has significantly contributed to the rising cases of neonatal pulmonary hypertension, thereby increasing the demand for inhaled nitric oxide. Growing awareness regarding the benefits of inhaled nitric oxide and strong clinical evidence supporting its efficacy are further expected to support the market growth during the forecast period.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
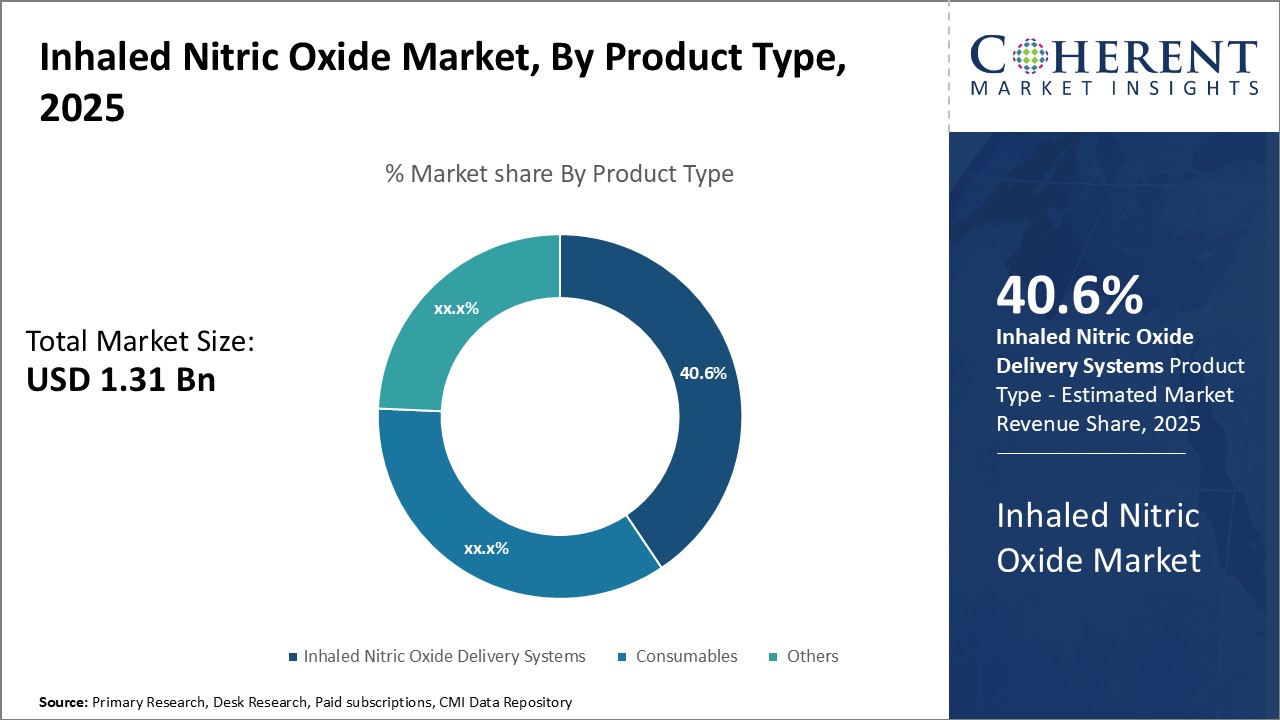
Insights By Product Type – Inhaled Nitric Oxide Delivery Systems Segment Leads with Growing Demand for Precise Delivery
The Inhaled Nitric Oxide Delivery Systems segment dominates the market with 40.6% in 2025 due to its critical role in treating severe respiratory conditions, particularly in neonates and patients with hypoxemic respiratory failure. These systems ensure precise and continuous delivery of nitric oxide, enhancing oxygenation, and reducing pulmonary hypertension. The increasing prevalence of respiratory diseases and advancements in portable delivery technologies further drive demand, making these systems essential in both hospital and outpatient settings
Insights By Application - Expanding Treatment Avenues Stimulate Neonatal Respiratory Segment Growth
The neonatal respiratory treatment segment is expected to lead the market with 35.62% share in 2025, driven by rising preterm birth rates and increased awareness of nitric oxide. According to WHO, 1 in 10 babies are born preterm, often facing respiratory complications. Inhaled nitric oxide therapy is the standard for preterm neonates, reducing issues like pulmonary hypertension. With higher survival rates and rising chronic lung diseases, hospitals are adopting nitric oxide therapies for respiratory health.
Insights By Strength - 100 ppm Dominates on Account of Established Efficacy and Dosing Flexibility
The 100 ppm segment is expected to lead the market with 46.62% share in 2025, due to its effectiveness in treating pulmonary hypertension across varying severities. Clinical evidence supports 100 ppm as optimal dosing for both neonatal and adult conditions while minimizing toxicity risks. Its flexibility in titration based on individual response and widespread use in hospitals for conditions like persistent pulmonary hypertension makes it the preferred choice over higher concentrations like 800 ppm.
Regional Insights
Need a Different Region or Segment? Download Free Sample
Regional Analysis:
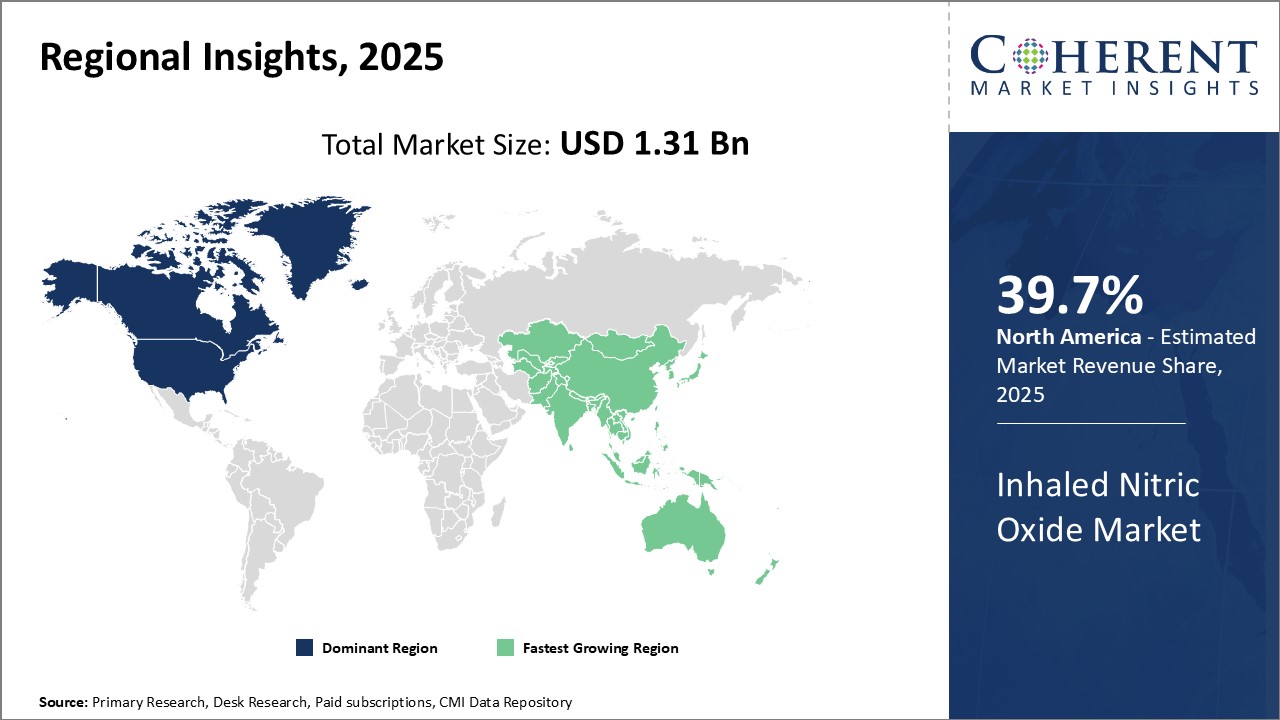
Dominating Region: North America
North America dominates the inhaled nitric oxide market with a 39.7% market share in 2025 due to its advanced healthcare infrastructure, extensive research and development, and the presence of key industry players. Supportive government policies, such as funding for innovation and regulatory frameworks, further enhance market growth, driving technological advancements and broad adoption of new treatment options in the region.
Fastest-Growing Region: Asia Pacific
The Asia Pacific region is the fastest-growing market for inhaled nitric oxide due to rising healthcare investments, increasing respiratory disease prevalence, expanding healthcare access, and growing awareness of inhaled Nitric Oxide benefits. These factors are driving the adoption of advanced medical therapies like inhaled nitric oxide, fueling the market growth in the region.
Inhaled Nitric Oxide Market Outlook for Key Countries
U.S.: Growth Driven by Robust Healthcare Infrastructure and Regulatory Support
The U.S. inhaled nitric oxide industry growth is fueled by advanced healthcare infrastructure and a high prevalence of respiratory disorders. Increased government initiatives supporting respiratory health further enhance the market growth, making the U.S. a key player in the global inhaled nitric oxide landscape. For instance, on February 9, 2025, a study by the University of Alabama found that high-dose inhaled nitric oxide therapy improved oxygenation and reduced mortality risk in critically ill Black COVID-19 patients with acute respiratory distress syndrome (ARDS) offering significant benefits over standard treatments for respiratory distress.
Canada: Boosted by Clinical Trials and Government Backing for Innovative Therapies
A rise in clinical trials and research activities drive the inhaled nitric oxide industry in Canada. Key drivers include robust healthcare infrastructure, government support for innovative therapies, and increasing demand for new respiratory treatments. For instance, in April 2020, Mallinckrodt and Novoteris received Health Canada's approval for a clinical trial using high-dose inhaled nitric oxide (iNO) therapy to treat COVID-19-related lung infections.
China: Expanding Healthcare Sector and Government Investment Propel Market Growth
China’s inhaled nitric oxide market is growing on the account of its expanding healthcare sector, increasing incidence of respiratory diseases, and rising government investment in advanced medical technologies. With a growing emphasis on enhancing healthcare infrastructure and regulatory support for innovative treatments, China is becoming a key player in the inhaled nitric oxide market.
Japan: Increased Use of iNO in Extremely Premature Infants Drives the Market Expansion
In Japan, the growth of the inhaled nitric oxide market is driven by its increased use in the treatment of extremely premature infants. For instance, in September 2022, according to Minute Medicine, Inc., a publishing organization, a study in Japan (2003-2016) showed an increase in the use of inhaled nitric oxide and survival rates in extremely premature infants. However, there were no differences in long-term neurological outcomes at age 3 between those treated with iNO and those who were not.
India: Proven Efficacy in COVID-19 Treatment Enhances iNO Market Position
In India, the inhaled nitric oxide market is expanding due to its proven efficacy in treating critical COVID-19 patients. A study conducted by Amrita Institute of Medical Sciences and Amrita Vishwa Vidyapeetham in February 2022 demonstrated that iNO reduced complications and resulted in zero mortality, outperforming standard treatments. This success, along with iNO’s established role in treating respiratory conditions, enhances its position as a vital therapy in India’s healthcare system, driving the market growth.
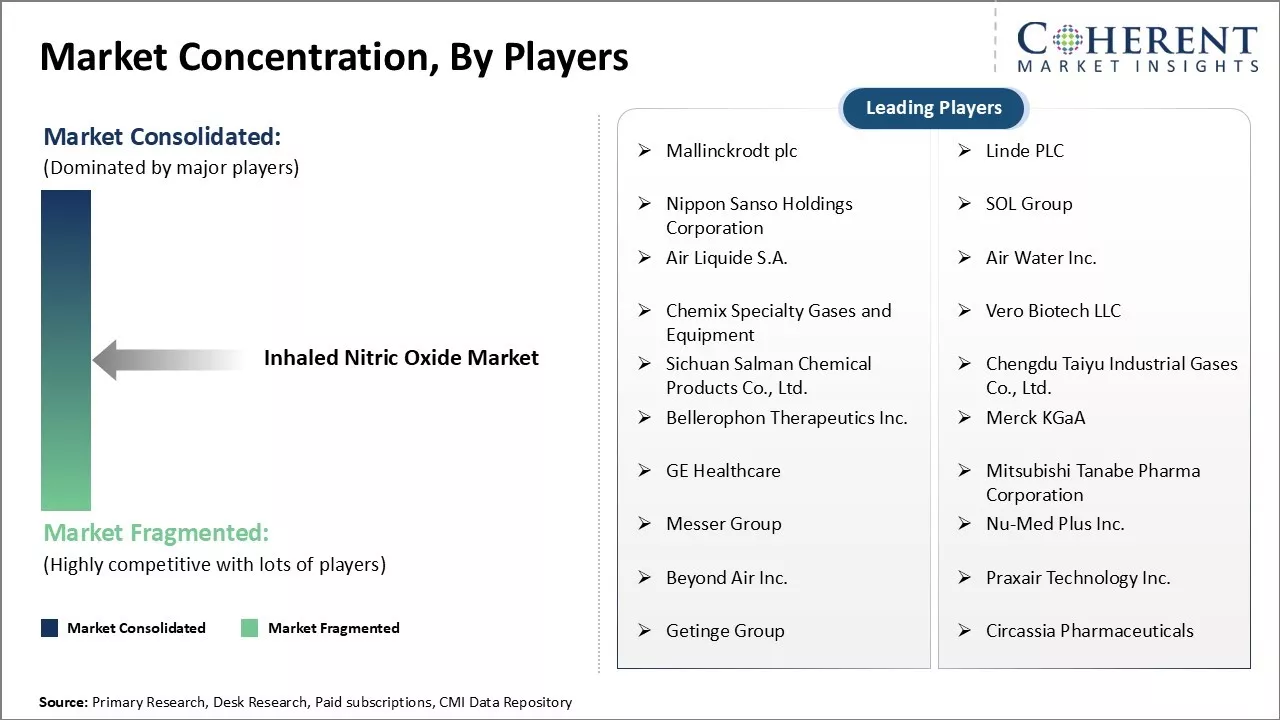
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Inhaled Nitric Oxide Market Players
- Established Players: Leading companies heavily invest in research and development to introduce advanced products. For example, IPSEN spends over 15% of its annual revenue on R&D to develop novel drug delivery systems and specialty pharmaceuticals for use in inhaled nitric oxide therapy. Major players also form strategic partnerships with other industry giants
- Mid-Level Players: Companies at this level focus on providing cost-effective solutions to attract price-sensitive customers. For example, Air Liquide produces generic inhaled nitric oxide concentrators at 30-50% lower costs than branded equivalents. They collaborate with local distributors and hospitals to promote their affordable products.
- Small-Scale Players: Small companies identify specialized therapeutic areas to occupy underserved niche markets. For instance, Beyond Air concentrated only on developing a solid-state inhaled nitric oxide generator for treating severe lung infections in infants.
Emerging Startups in the Inhaled Nitric Oxide Market
- Innovative Technologies: Numerous startups are focusing on advanced technologies like Vero Biotech which is developing smart sensor-based nitric oxide delivery systems powered by AI algorithms. Their smart NO delivery platform could transform existing treatment methods by precisely adjusting dosage in real-time.
- Sustainable Solutions: Some startups deliver eco-friendly options - Ekosia produces recyclable concentrators using 70% recycled plastics. They aim to reduce dependence on single-use equipment which generates 1,200 tons of medical waste annually. Ekosia's sustainable model promotes a greener respiratory care industry.
- Market Contribution: Startups often address unavailable or underserved market segments. For example, Nu-Ox focused solely on developing an ultra-portable inhaled nitric oxide generator for use in emergency transport situations. They partnered with various ambulance services to boost accessibility of the life-saving therapy during transport. Such collaborations facilitate startups in overcoming commercialization hurdles.
Key Takeaways from Analyst
- The inhaled nitric oxide market is expected to grow due to rising preterm births and increasing use in treating respiratory conditions. North America leads the market, while the Asia Pacific region is poised for rapid growth due to higher healthcare spending and expanding infrastructure. Challenges include high treatment costs and expensive delivery systems. Opportunities lie in targeting emerging markets, innovating delivery equipment to reduce costs, and broadening clinical indications.
Market Report Scope
Inhaled Nitric Oxide Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.31 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.7% | 2032 Value Projection: | USD 2.21 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Mallinckrodt plc, Linde PLC, Nippon Sanso Holdings Corporation, SOL Group, Air Liquide S.A., Air Water Inc., Chemix Specialty Gases and Equipment, Vero Biotech LLC, Sichuan Salman Chemical Products Co., Ltd., Chengdu Taiyu Industrial Gases Co., Ltd., Bellerophon Therapeutics Inc., Merck KGaA, GE Healthcare, Mitsubishi Tanabe Pharma Corporation, Messer Group, Nu-Med Plus Inc., Beyond Air Inc., Praxair Technology Inc., Getinge Group, and Circassia Pharmaceuticals |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Dynamics
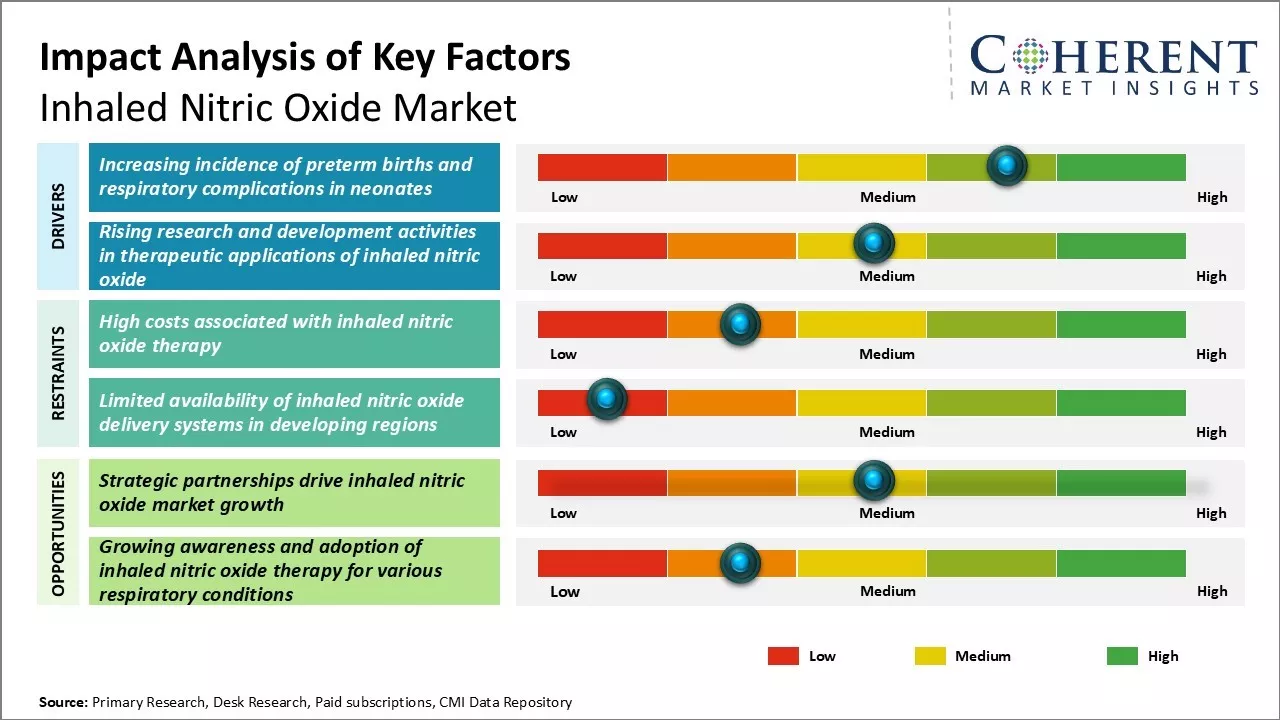
Market Driver - Increasing incidence of preterm births and respiratory complications in neonates
The incidence of preterm birth has been steadily increasing in both developed and developing countries, with significant implications for neonatal health. For instance, according to a WHO report in May 2023, the rise in preterm births correlates with higher rates of respiratory complications in neonates, such as pulmonary hypertension and respiratory failure. This situation has heightened the demand for effective treatments like inhaled nitric oxide (iNO) therapy, which is crucial for managing conditions associated with respiratory distress syndrome due to surfactant deficiency.
Market Challenge - High costs associated with inhaled nitric oxide therapy
A major challenge in the inhaled nitric oxide market is the high cost of therapy, which includes complex delivery systems, gas handling equipment, and expensive gas itself. Annual costs per patient range from US$ 30,000 to US$ 50,000, with one treatment averaging over US$ 1,000. This financial burden limits adoption, especially in price-sensitive markets. Reducing equipment and operational costs through innovation is crucial to making INO therapy more accessible and driving long-term market growth.
Market Opportunity- Strategic partnerships drive inhaled nitric oxide market growth
Strategic partnerships in the inhaled nitric oxide market offer substantial growth opportunities by integrating expertise, resources, and technologies to enhance product portfolios. For instance, October 2, 2024, Beyond Air, a medical device and biopharmaceutical company, partnered with Healthcare Links, a healthcare advisory and contracting firm, to expand the availability of its LungFit PH system, a groundbreaking inhaled nitric oxide (NO) generator and delivery system.
Key Stakeholders of Market
What Growth in Inhaled Nitric Oxide Market Mean for Different Stakeholders?
The inhaled nitric oxide market has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Pharmaceutical Stakeholder |
Opportunities Due to Inhaled Nitric Oxide Industry Growth |
|
Retail Pharmacies |
Expansion of product offerings to include new drugs and personalized medicine solutions, enhancing customer care and market reach. |
|
Chemical Suppliers |
Growth in demand for specialty chemicals used in drug synthesis, including organic intermediates, catalysts, and reagents. |
|
Pharmaceutical Companies |
Expansion of product pipelines with new drug discoveries, biologics, and biosimilars, capitalizing on growing global healthcare needs. |
|
Contract Research Organizations (CROs) |
Increased outsourcing of clinical trials and drug development, offering opportunities for growth and long-term partnerships. |
|
Contract Manufacturing Organizations (CMOs) |
Growing demand for scalable manufacturing solutions, including biologics production and complex drug formulations. |
|
Raw Materials Suppliers |
Increased demand for high-quality active pharmaceutical ingredients (APIs) and excipients to support drug formulation and production. |
|
Healthcare Providers |
New treatment options and innovative therapies, improving patient care and expanding healthcare services. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Segmentation
- Product Type Insights (Revenue, USD Bn, 2020 - 2032)
-
- Inhaled Nitric Oxide Delivery Systems
- Consumables
- Nitric Oxide Cylinders
- Delivery Accessories
- Others
- Application Insights (Revenue, USD Bn, 2020 - 2032)
-
- Neonatal Respiratory Treatment
- Chronic Obstructive Pulmonary Disease (COPD)
- Acute Respiratory Distress Syndrome (ARDS)
- Tuberculosis Treatment
- Malaria Treatment
- Others
- Strength Insights (Revenue, USD Bn, 2020 - 2032)
-
- 100 ppm
- 800 ppm
- Others
- End User Insights (Revenue, USD Bn, 2020 - 2032)
-
- Hospitals
- Ambulatory Surgical Centers
- Home Care Settings
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Mallinckrodt plc
- Linde PLC
- Nippon Sanso Holdings Corporation
- SOL Group
- Air Liquide S.A.
- Air Water Inc.
- Chemix Specialty Gases and Equipment
- Vero Biotech LLC
- Sichuan Salman Chemical Products Co., Ltd.
- Chengdu Taiyu Industrial Gases Co., Ltd.
- Bellerophon Therapeutics Inc.
- Merck KGaA
- GE Healthcare
- Mitsubishi Tanabe Pharma Corporation
- Messer Group
- Nu-Med Plus Inc.
- Beyond Air Inc.
- Praxair Technology Inc.
- Getinge Group
- Circassia Pharmaceuticals
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
