Ingestible Sensors Market Size and Trends To 2025-2032
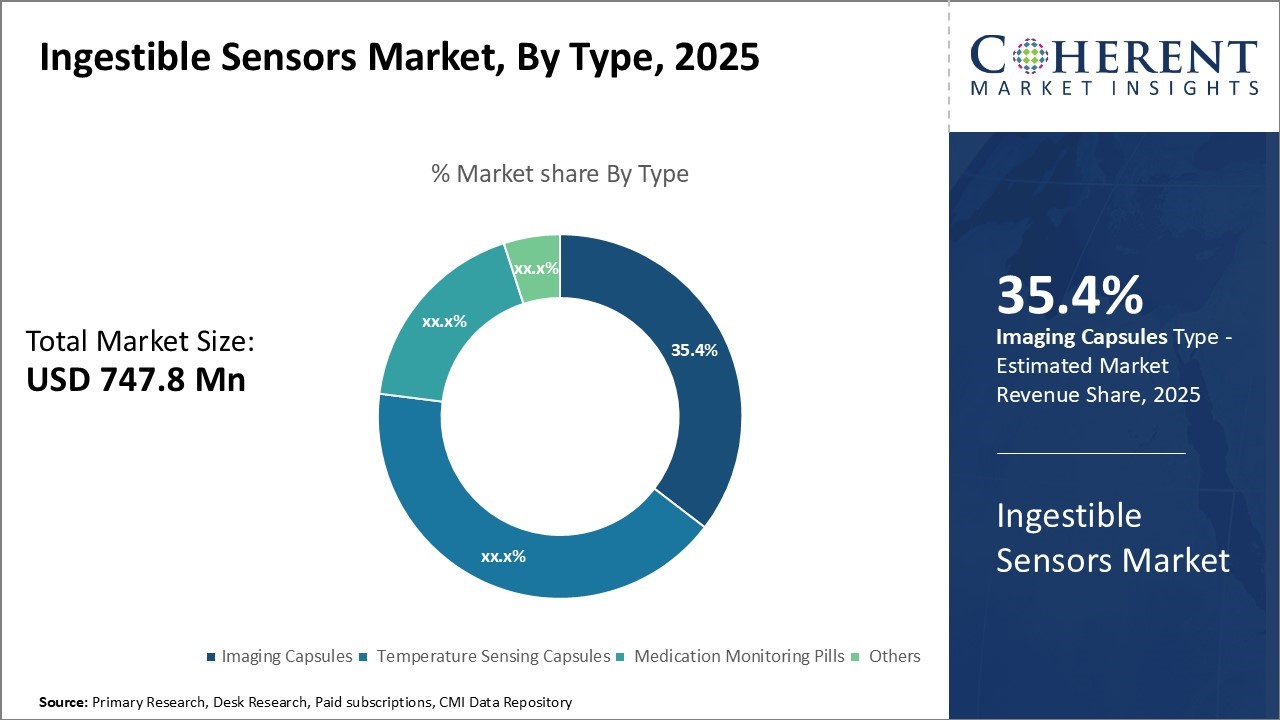
The global ingestible sensors market is estimated to be valued at USD 747.8 Mn in 2025 and is expected to reach USD 1,826.8 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 13.6% from 2025 to 2032.
To learn more about this report, Download Free Sample
Key Takeaways
- Based on Type, Imaging Capsule segment is projected to account for 35. 4% of the global market in 2025, owing to it’s their non-invasive nature and ability to capture high quality images
- Based on End User, Healthcare segment is projected to command 33.6% share of the market in 2025, due to growing emphasis on non-invasive diagnosis and monitoring within the industry.
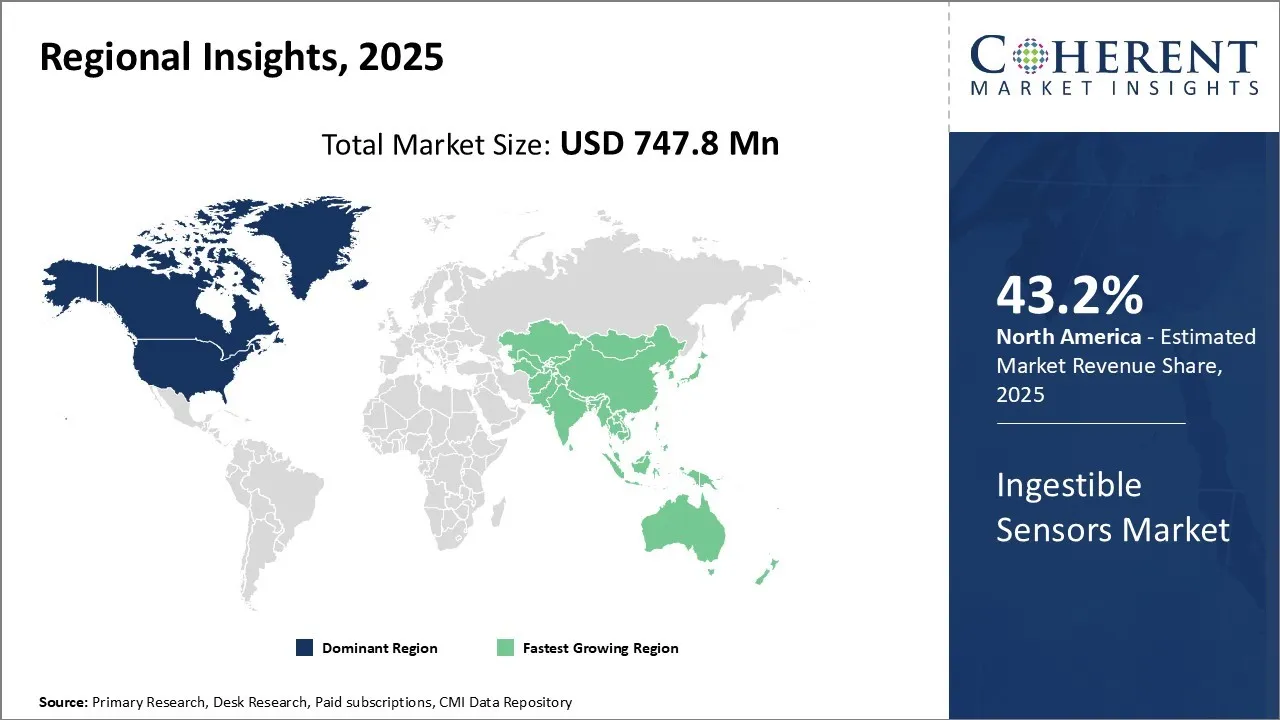
- Based on Region, North America is set to dominate the global market with a 43.2% share in 2025.
Market Overview
The rising prevalence of chronic diseases and the escalating demand for continuous monitoring of health-related parameters are anticipated to enhance the adoption of ingestible sensors. Also, improvements in microelectromechanical (MEMS) and wireless technologies have made it possible to make small, precise ingestible sensors. This has made them more useful for keeping an eye on different bodily functions that affect gut health, endoscopy, drug delivery, and nutrition management.
Also, increasing investments by market players for the development of novel ingestible sensor-based devices and government support are further expected to drive the market growth during the forecast period.
Current Events and their Impact
|
Current Event |
Description and its Impact |
|
FDA and Global Regulatory Framework Evolution |
|
|
Global Aging Population and Chronic Disease Management Crisis |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Role of AI (Artificial Intelligence) in Ingestible Sensors Market
The combination of artificial intelligence and ingestible sensor technology is changing the way healthcare is monitored and opening up new possibilities for personalized medicine and real-time health tracking. AI is changing the way these pill-sized devices gather, process, and understand biological data from inside the human body as they get better.
For instance, in August 2024, researchers at the USC Viterbi School of Engineering developed advanced ingestible sensors that utilize AI and wearable electronics to provide real-time 3D monitoring of gastrointestinal health. These innovative smart pills can detect stomach gases and track their location within the body, offering potential for early disease detection.
Market Drivers
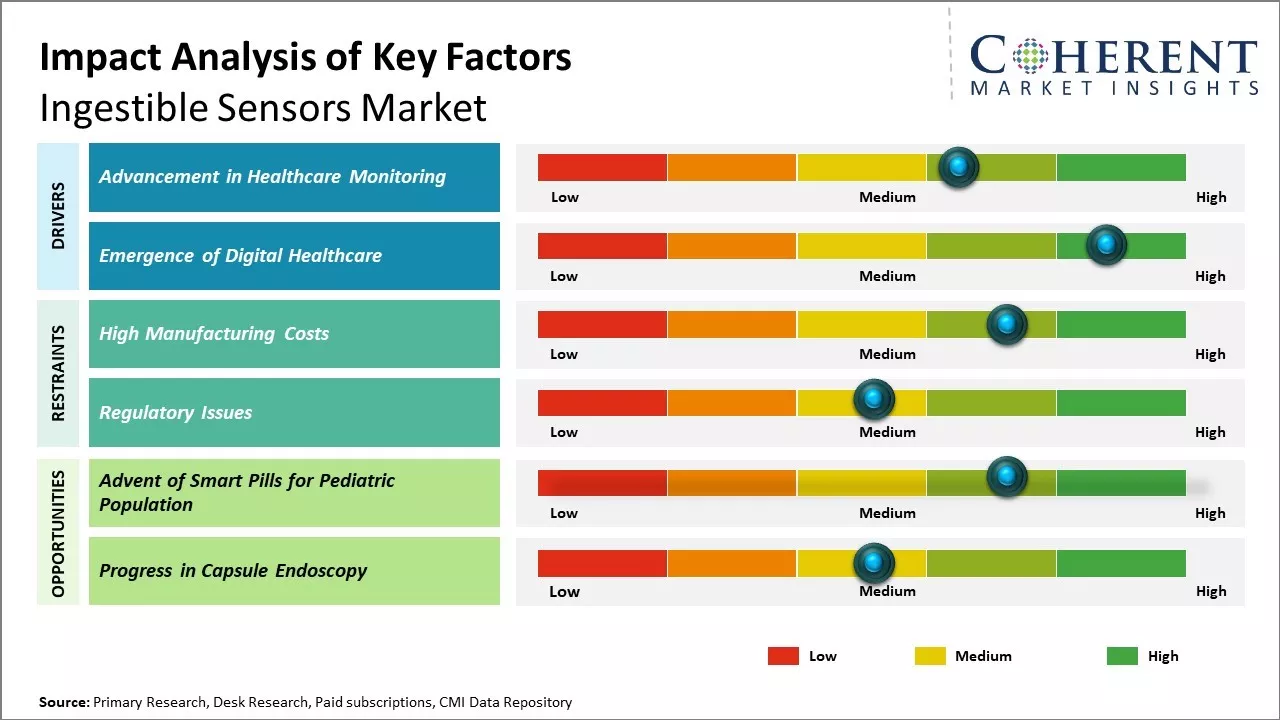
Advancement in Healthcare Monitoring
The global population is aging at an unprecedented rate which has increased the burden on healthcare systems worldwide. This has fueled the demand for innovative diagnostic and monitoring solutions that can help provide care for the growing elderly population. Ingestible sensors are revolutionizing how care can be provided as they allow for continuous monitoring of vital health parameters from inside the body in a minimally invasive manner.
Emergence of Digital Healthcare
Digital technologies have transformed various aspects of modern life and healthcare is one sector that has been experiencing a paradigm shift. There is a growing trend of patients actively taking charge of their health and wellness through self-monitoring devices and apps. This has been driven by the ubiquitous availability of smartphones and other connected devices.
This has created opportunities for digital health solutions like ingestible sensors that can facilitate virtual care. As patients seek contactless options, these sensors are well-positioned to monitor health from home in real-time.
Ingestible Sensors Market Insights, By Type: Advancements in Endoscopy Technology
The imaging capsules sub-segment is estimated to hold 35.4% of the market share in 2025. Imaging capsules have seen rising adoption rates in recent years due to their non-invasive nature and ability to capture high quality images from anywhere within the gastrointestinal tract.
As these capsules pass through the digestive system, they are able to transmit thousands of images wirelessly to monitoring devices worn by patients. This allows physicians to detect conditions like celiac disease, Crohn's disease, and tumors without the discomfort of traditional endoscopic procedures. Technological improvements have played a key role in driving the growth of imaging capsules.
Newer capsules are equipped with higher resolution cameras and longer battery lives, enabling clearer diagnosis. Some capsules can stay active for up to 12 hours after ingestion. Advancements in wireless transmission have also increased data transfer speeds and image quality. Miniaturization trends continue to make capsules smaller and easier to swallow. New SmartPill capsules even incorporate pH, pressure and temperature sensors in addition to imaging.
For instance, in February 2023, Engineers at MIT and Caltech have demonstrated an ingestible sensor whose location can be monitored as it moves through the digestive tract, an advance that could help doctors more easily diagnose gastrointestinal motility disorders such as constipation, gastroesophageal reflux disease, and gastroparesis.
Ingestible Sensors Market Insights, By End-use - Need for Non-invasive Diagnostics
The healthcare sub-segment is estimated to hold 33.6% of the market share in 2025 due to the growing emphasis on non-invasive diagnosis and monitoring within the industry. Conditions affecting the digestive health are highly prevalent but often require examination of the entire gastrointestinal tract. Imaging capsules and other ingestible sensors address this need by enabling visualization from inside without surgical procedures.
They significantly reduce discomfort for patients and procedure time for clinicians compared to endoscopies. This improves diagnostics for troublesome issues like internal bleeding, ulcers and inflammation. Healthcare providers are also under constant pressure to enhance efficiency and reduce costs.
Ingestible sensors help optimize resource usage within hospitals and diagnostic centers. They can be administered by trained nurses rather than specialists, freeing up expert time. Early detection of issues via frequent mass screening also cuts down on future advanced treatment requirements.
Regional Insights
To learn more about this report, Download Free Sample
North America Ingestible Sensors Market Analysis & Trends
North America remains the dominant region in the global ingestible sensors market and is estimated to hold 43.2% of the market share in 2025. The region has a well-established healthcare infrastructure with large healthcare spending per capita. Many of the leading technology companies involved in developing ingestible sensor technologies are headquartered in the U.S.
This has created a strong innovation ecosystem which drives rapid advancements in the space. Additionally, favorable reimbursement policies and awareness among healthcare providers as well as consumers has led to high adoption rates of these next generation medical devices. The region is also a major exporter of these devices to other developed markets. However, pricing pressures from payers are forcing companies to look at reducing production costs.
For instance, in July 2025, an innovative gas-sensing capsule developed at RMIT University to provide real-time insights into gut health has received regulatory clearance from the US Food and Drug Administration (FDA), paving the way for its commercial launch in the United States.
Asia Pacific Ingestible Sensors Market Analysis & Trends
The Asia Pacific region has emerged as the fastest growing market for ingestible sensors in recent years. Countries such as China, India, Japan, and South Korea are contributing significantly to the Asia Pacific market growth. This growth can be attributed to factors like rising healthcare expenditure, growing medical tourism industry, and increasing occurrence of chronic diseases in the region. The large patient pool and focus on developing home healthcare solutions are further propelling the Asia Pacific ingestible sensors market.
The presence of several contract manufacturing organizations has made this region an attractive production base for global players looking to reduce overhead costs. Regional players are also making efforts to develop low-cost sensor solutions tailored for the price-sensitive APAC markets. Exporting to other developing Asian and African countries present lucrative opportunities. The market is expected to continue on its high growth trajectory in the coming years.
Ingestible Sensors Market Outlook Country-Wise
The U.S. Ingestible Sensors Market Trends
The U.S. ingestible sensors market is experiencing significant growth, driven by advancements in healthcare technology and increasing demand for personalized medicine. These sensors, often referred to as "digital pills," are embedded within medications to monitor patient adherence and physiological responses.
China Ingestible Sensors Market Trends
China's ingestible sensors market is experiencing robust growth, driven by advancements in digital health technologies and increasing demand for personalized healthcare solutions.
Market Concentration and Competitive Landscape
To learn more about this report, Download Free Sample
Analyst Opinion (Expert Opinion)
- The global ingestible sensor market is poised for significant growth in next five years. One of the major drivers fueling growth is increasing usage of these sensors in medical applications to monitor physiological parameters for various diseases and conditions in chronic settings. The rising focus on preventive healthcare and remote patient monitoring is expected to further propel demand.
- North America is projected to continue dominating ingestible sensor market owing to large presence of leading manufacturers and favorable regulations. However, Aisa Pacific is likely to emerge as the fastest growing regional market due to rising health expenditure and growing medical tourism industry.
Ingestible Sensors Industry News
- In July 2025, A breakthrough in gastrointestinal health monitoring was unveiled as nanoelectronics specialist ImecSearch company demonstrated a highly miniaturized ingestible sensor at the ITF World conference.
- In April 2025, Atmo Biosciences, a leader in ingestible capsule technology, announced the successful assignment of its foundational patent portfolio from RMIT University. Under this agreement, RMIT has transferred all patents and associated intellectual property (IP) related to the Atmo Gas Capsule to Atmo Biosciences in exchange for an equity stake in the company.
- In April 2022, AARDEX announced about its strategic partnership with the etectRx Inc., that combines the etectRx’s ID-Cap ingestible System and AARDEX’s MEMS Adherence Software to track the medication-taking behaviors. They both will provide a precise and holistic method for measuring adherence to solid form dose medications.
- In March 2022, Royal Philips, unveiled Azurion System with FlexVision & Ambient Experience. The new technology is mainly designed to allow the members of interventional team to easily adapt to room’s ambient lighting & sound for improving the overall patient & staff experience.
Market Report Scope
Ingestible Sensors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 747.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.6% | 2032 Value Projection: | USD 1,826.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
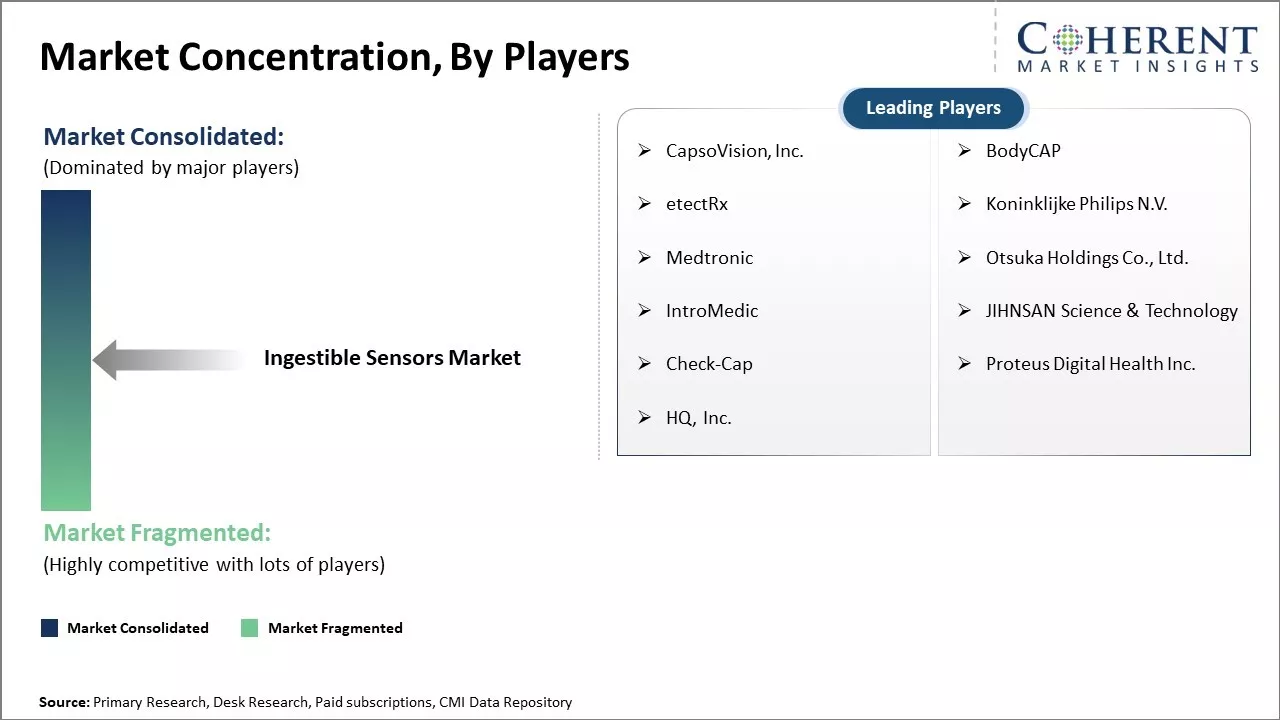
CapsoVision, Inc., BodyCAP, etectRx, Koninklijke Philips N.V., Medtronic, Otsuka Holdings Co., Ltd., IntroMedic, JIHNSAN Science & Technology, Check-Cap, Proteus Digital Health Inc., and HQ, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Opportunities – Progress in Capsule Endoscopy
Capsule endoscopy involves patients swallowing a small camera capsule that takes thousands of pictures of the digestive tract as it travels through. This non-invasive procedure allows physicians to detect diseases like celiac disease, Crohn's disease, and small intestinal cancers more effectively compared to other usual diagnostic tests.
The technology behind capsule cameras has advanced significantly in recent years, with improvements in image resolution, larger data storage capacity and options for control over the camera. Newer capsule endoscopes feature magnets that allow doctors to control the movement of the capsule using an external device. This provides the ability to stop, start or change the direction of the capsule when required.
Some capsules can also release medications at targeted locations or take biopsies using detachable multipurpose tools. With continuing technological developments, future capsules may be embedded with sensors to detect biomarkers in real-time and transmit the results. This would enable continuous monitoring of diseases and timely interventions. The decreasing size of electronic components has made it possible to pack more advanced capabilities into tiny ingestible sensors.
Market Segmentation
- Type Insights (Revenue, USD Mn, 2020 - 2032)
- Imaging Capsules
- Temperature Sensing Capsules
- Medication Monitoring Pills
- Others
- End-use Insights (Revenue, USD Mn, 2020 - 2032)
- Healthcare
- Sports & Fitness
- Others
- Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- CapsoVision, Inc.
- BodyCAP
- etectRx
- Koninklijke Philips N.V.
- Medtronic
- Otsuka Holdings Co., Ltd.
- IntroMedic
- JIHNSAN Science & Technology
- Check-Cap
- Proteus Digital Health Inc.
- HQ, Inc.
Sources
Primary Research Interviews
- Medical Device Manufacturers
- Healthcare Technology Companies
- Digital Health Solution Providers
- Pharmaceutical Companies
- Others
Databases
- FDA Medical Device Database
- GlobalData Healthcare Intelligence
- Frost & Sullivan Healthcare Database
- Others
Magazines
- Medical Device and Diagnostic Industry (MDDI)
- Healthcare IT News
- Digital Health Magazine
- Medical Electronics Design
- Others
Journals
- Nature Biomedical Engineering
- IEEE Transactions on Biomedical Engineering
- Journal of Medical Internet Research
- Others
Newspapers
- The Wall Street Journal
- Financial Times
- Reuters Health News
- Healthcare Finance News
- Others
Associations
- Medical Device Manufacturers Association (MDMA)
- Healthcare Information and Management Systems Society (HIMSS)
- Digital Medicine Society (DiMe)
- International Association of Medical Device Regulators (IAMDR)
- Others
Public Domain Sources
- U.S. Food and Drug Administration (FDA)
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- National Institutes of Health (NIH)
- Other
Proprietary Elements
- CMI Data Analytics Tool
- Proprietary CMI Existing Repository of information for last 8 years
*Definition: Ingestible sensors are electronic devices or sensors in the form of pills or capsules that can be swallowed and pass through the gastrointestinal tract. These ingestible sensors once ingested can transmit physiological and environmental data from inside the body to an external monitoring device via wireless communication. They help in monitoring bodily functions and detecting medical conditions from within the body without any surgical procedures.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients