Incretin Mimetics Market Size and Trends
The incretin mimetics market is estimated to be valued at USD 23.84 Bn in 2025 and is expected to reach USD 33.56 Bn by 2032, growing at a compound annual growth rate (CAGR) of 5.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
This class of drugs helps control blood sugar levels in patients with type 2 diabetes by mimicking the effects of incretin hormones like GLP-1. These medications stimulate insulin secretion and inhibit glucagon secretion in a glucose-dependent manner.
The market’s growth is driven by the rising global prevalence of diabetes. Incretin mimetics reduce cardiovascular risks and promote weight loss, offering an effective treatment option for overweight patients with diabetes. However, the availability of alternative drug classes and cost concerns could restrict market growth to some extent in the forecast period.
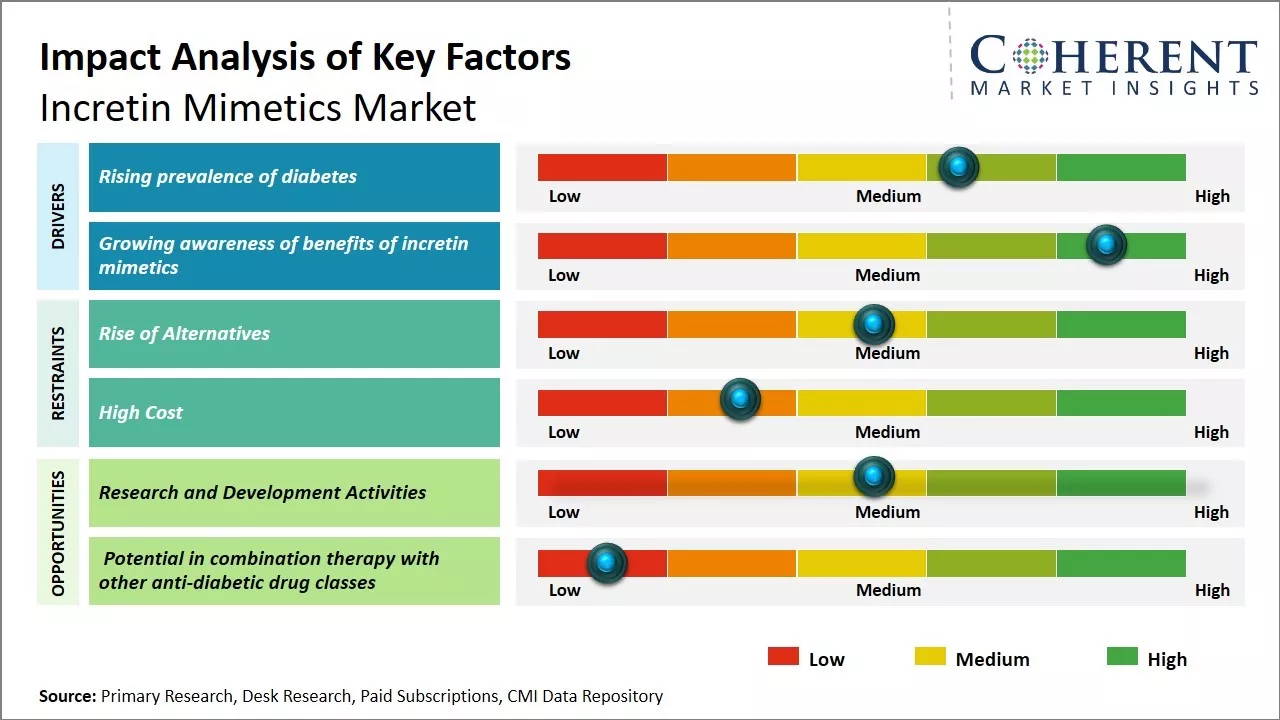
Rising prevalence of diabetes
The global prevalence of diabetes has been steadily rising. According to the IDF Diabetes Atlas, in 2021, over 537 million adults (20-79 years old) were living with diabetes. This number is predicted to rise to 643 million by 2030 and 783 million by 2045. While Type 2 diabetes accounts for the majority of diabetes cases worldwide, the incidence of Type 1 diabetes is also increasing at an alarming rate. The rising global burden of diabetes has major public health and economic repercussions. Not only does diabetes significantly increase the risk of debilitating and potentially fatal medical complications such as heart disease, stroke, kidney failure, blindness and lower limb amputations, but it also leads to tremendous direct healthcare costs and indirect costs related to lost productivity and work absenteeism. With the number of diabetes cases continuing to grow each year across all regions of the world, there is heightened awareness about the importance of glucose control in diabetes management and the need for advanced therapeutic options. More physicians and patients are recognizing the value of newer anti-diabetic drugs like incretin mimetics that can help improve glycemic control with a lower risk of hypoglycemia and weight gain compared to older drug classes.
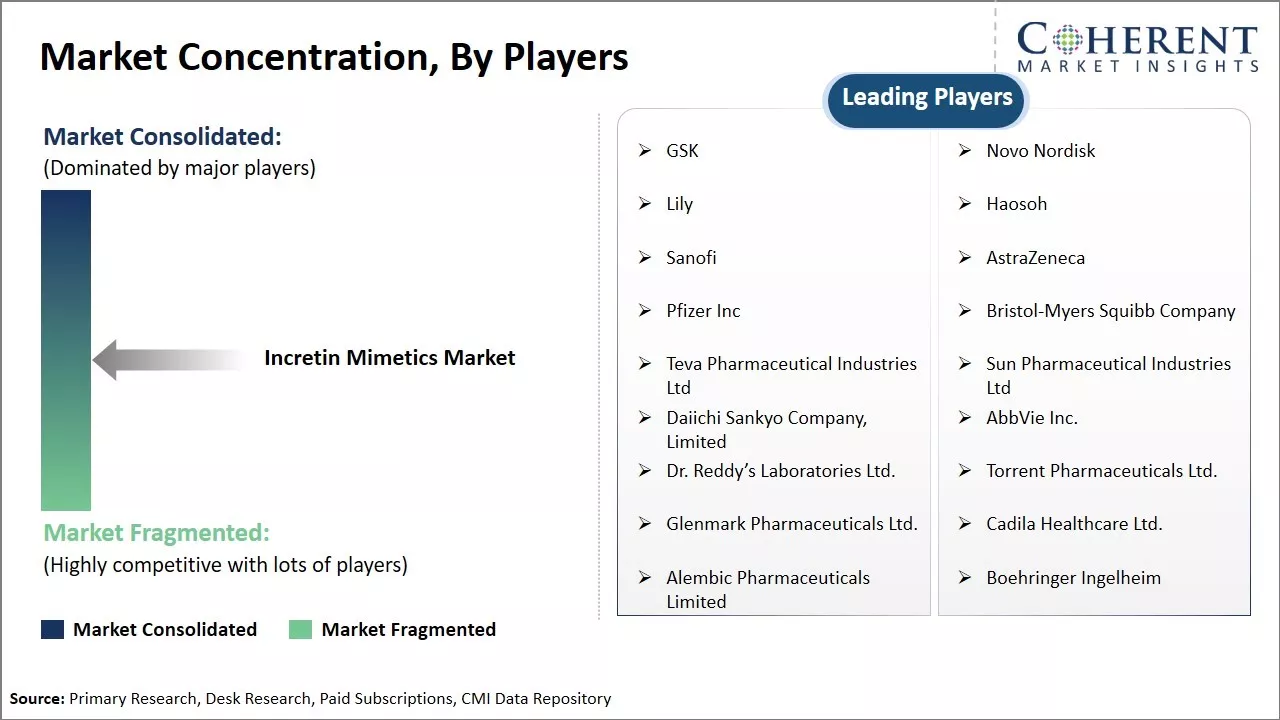
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Growing awareness of benefits of incretin mimetics
With incretin mimetics having been on the market for over a decade, there is significant awareness within the medical community as well as among patients about the clinical profile and advantages of this class of anti-diabetes drugs. Extensive clinical research has demonstrated the ability of incretin mimetics to reduce HbA1c levels while minimizing the risks of hypoglycemia and weight gain that are commonly associated with other glucose-lowering medications. Post-marketing studies have also established their cardiovascular safety. These key benefits compared to older drugs have generated much interest among physicians who prefer to initiate patients on incretin mimetics as first-line injectable therapy when oral drugs are not sufficient. Additionally, the risk and complications associated with hyperglycemia and poorly controlled diabetes are now better appreciated by patients. They are more proactively involved in managing their condition and open to therapies that can help achieve optimal glucose control safely.

To learn more about this report, Download Free Sample
Market Challenges: Rise of Alternatives
The incretin mimetics market faces several challenges in further growth. As newer treatment options emerge for type 2 diabetes, some incretin mimetics face increasing competition. The delivery methods of some drugs have faced patient adherence challenges. Further, the patent expirations of major brands in the next 2-3 years could enable the entry of cheaper generics, exerting downward pressure on prices.
Market Opportunities: Research and Development Activities
Research and development activities in the area of incretin mimetics present a significant opportunity for growth in this market. Incretin mimetics are a class of drugs that mimic the effects of natural incretins like GLP-1 and GIP. These important gastrointestinal hormones play a key role in regulating important processes like glucose-dependent insulin secretion, suppression of glucagon secretion, delay in gastric emptying, etc.
By focusing more resources on research and development, pharmaceutical companies can develop more advanced incretin mimetic drugs with improved efficacy, safety, and novel delivery mechanisms. Currently available drugs in this class, like exenatide and liraglutide have revolutionized diabetes treatment. However, long term adherence can be challenging due to factors like frequent injections. R&D can help address these limitations by coming up with newer oral or non-injectable formulations of incretin mimetics. Several companies already have novel drug candidates in the late stages of clinical trials that offer weekly or monthly dosing options instead of daily injections.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
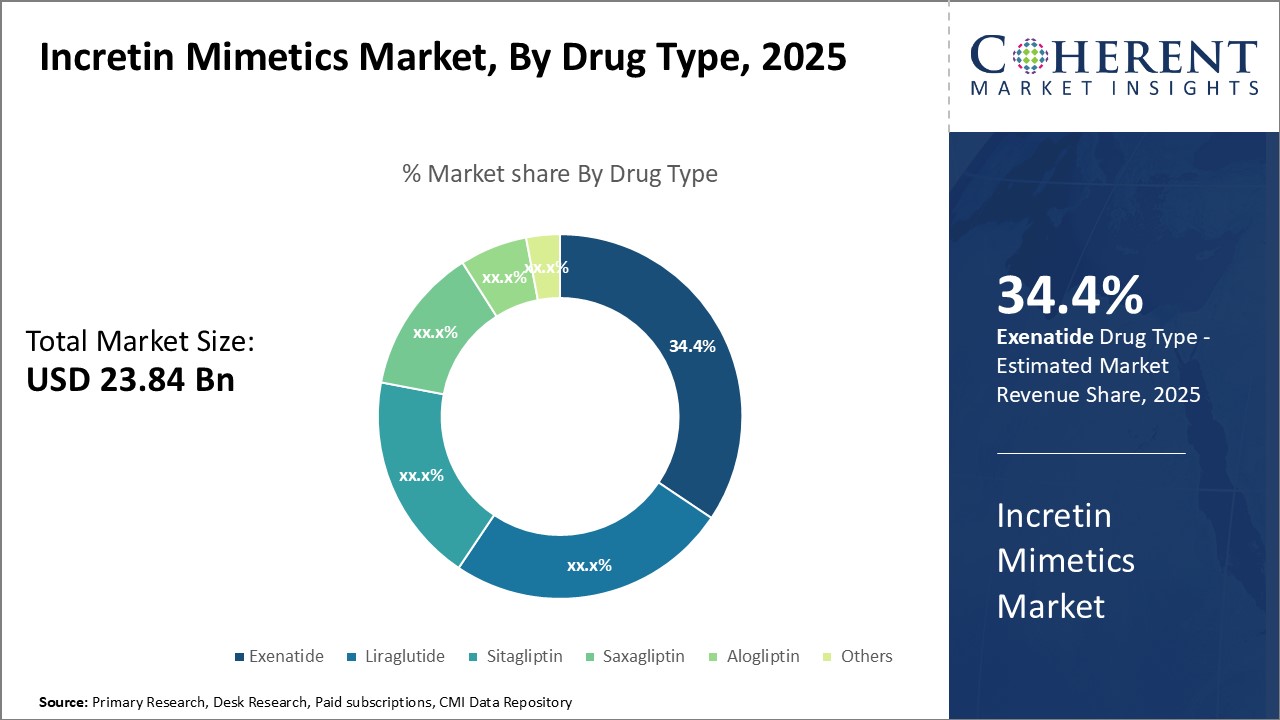
Insights, By Drug Type: Efficacy and advantages over other agents drives Exenatide’s growth
The drug type segment includes exenatide, liraglutide, sitagliptin, saxagliptin, alogliptin, and others. Exenatide is anticipated to register 34.4% of the market share in 2024. As one of the earliest DPP-4 inhibitors introduced in the market, it has established itself as an important treatment option for type 2 diabetes management. Exenatide is well-tolerated and offers robust glycemic control for patients. Its distinct GLP-1 receptor agonist properties help promote satiety and weight loss, offering additional benefits beyond baseline glucose control.
Exenatide provides superior control over post-prandial hyperglycemia compared to conventional therapies due to its ability to mimic the effects of endogenous incretin hormones. This delivers added benefits in managing elevated glucose levels following meals. Further clinical evidence also indicates that long-term therapy with exenatide may offer some degree of pancreatic β-cell protection, an important consideration in slowing disease progression.
The availability of both short and long-acting formulations allows for flexible dosing regimens depending on patient needs. This enhances medication compliance and treatment effectiveness over time. Both formulations are generally well-tolerated with few serious side effects reported in long-term follow up studies. Overall, exenatide presents a highly cost-effective alternative to other antidiabetic agents for individuals seeking optimal glycemic control along with weight management support.
Insights, By Disease Indication: High prevalence drives Type 2 Diabetes Mellitus (T2DM) growth
The disease indication segment consists of Type 2 Diabetes Mellitus (T2DM), weight management, and others. Type 2 diabetes mellitus (T2DM) is anticipated to have the highest share, which is 43.2% of the market in 2025. This is primarily due to the high and growing prevalence of T2DM globally. T2DM typically develops in adulthood and is caused by the body's inability to properly use or produce enough insulin. The number of adults diagnosed with diabetes has almost quadrupled since 1980, rising from 108 million to 422 million in 2014, according to the World Health Organization. The Centers for Disease Control and Prevention estimates over 30 million Americans currently have diabetes, with the condition's economic burden in the US exceeding US$ 327 Bn annually. Other major markets like China and India have also seen significant rises in T2DM cases, driven by increasing obesity rates and aging populations.
The sizable and expanding T2DM patient pool drives demand for innovative drug therapies to improve blood sugar control. Incretin mimetics address a key deficiency in T2DM by mimicking the effects of hormones called incretins, which stimulate insulin secretion after meals. By supplementing this lacking incretin response, these agents help regulate blood glucose levels with fewer hypoglycemic risks than other diabetic medication classes.
Additionally, many incretin mimetics have demonstrated cardiovascular benefits in clinical trials. As diabetes substantially increases the risks of heart attack, stroke and other cardiac issues, obtaining proven heart protection offers a major treatment advantage.
Given diabetes' tremendous health and economic impact, new therapeutics that can better manage blood glucose with an improved safety profile will continue being priority treatment options for physicians and patients alike. This ensures the large T2DM segment remains the primary growth driver within the overall incretin mimetics market.
Insights, By Distribution Channel: Specialist management of incretin mimetics drives Hospital Pharmacies development
The distribution channel segment includes hospital pharmacies, retail pharmacies, and online pharmacies. Hospital pharmacies is anticipated to have the largest share of 35.3% of the market in 2025. This is primarily because incretin mimetics cater to chronic, complex conditions like T2DM that often require specialist involvement and management.
Many patients first receive incretin mimetic prescriptions during inpatient care following diabetes diagnosis or complications. Hospitals also provide supplemental education on medication administration and safety monitoring that may be especially critical in the case of newly prescribed injectable incretin therapies.
Outpatient drug coverage for conditions like diabetes is commonly provided through health insurance plans negotiated between hospitals, pharmacy benefits managers and insurance providers. This streamlines dispensation and reimbursement of incretin mimetics prescribed during follow-up specialist and primary care visits at affiliated hospital-run clinics.
Community endocrinologists and primary care physicians also favor prescribing to hospital pharmacies in some cases due to the latter's direct engagement with diabetes educators, nutritionists and other specialists. This multidisciplinary management approach coordinated through a single pharmacy maximizes therapy adherence and outcomes.
The need for specialized multichannel support means hospital pharmacies serving resident physicians and outpatients will likely maintain the lead in the incretin mimetics distribution landscape. Their integrated care model ensures optimal drug safety and efficacy for high-risk patients.
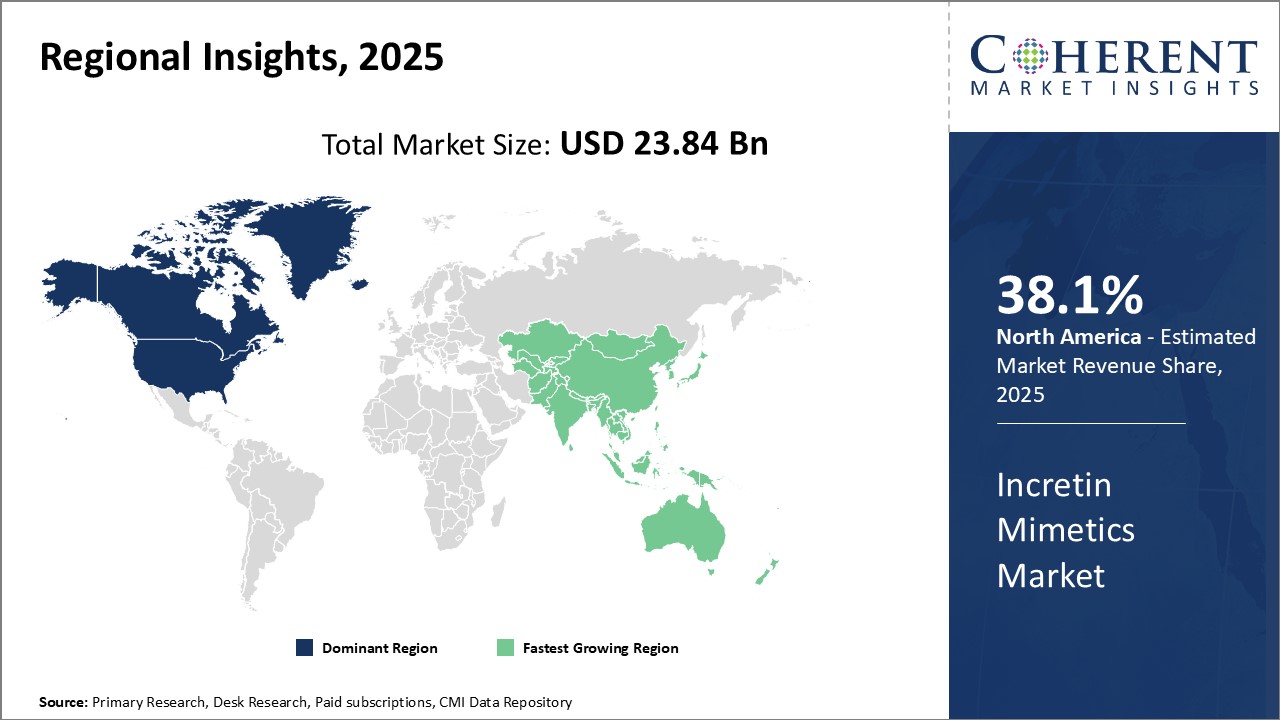
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America has emerged as the dominant region in the global incretin mimetics market. The U.S. accounts for the largest share, primarily due to growing awareness about diabetes management and rising adoption of GLP-1 receptor agonists and DPP-4 inhibitors. The presence of majority of the leading manufacturers, such as Novo Nordisk, Eli Lilly, and AstraZeneca, has also boosted drug availability and clinical research activities in the region. In addition, favorable reimbursement policies for diabetes drugs and rising healthcare spending contribute to the sales of these drugs.
The Asia Pacific region is poised to witness the fastest growth over the forecast period. Rapid urbanization, changing lifestyles, and rising geriatric population have significantly increased the prevalence of diabetes in countries like China and India. This has augmented the clinical need for effective diabetes management. Increasing healthcare expenditures of governments and availability of low-cost generic drugs are supporting the widespread use of incretin mimetics in disease treatment. Moreover, growing medical tourism across Asian countries is attracting many international players to strengthen their presence through collaborations with local players.
Europe holds a substantial share in the market led by countries such as Germany, the U.K., and France. High acceptance of new drug therapies, presence of major drug manufacturers and rising awareness among physicians regarding the benefits of incretin-based therapies have been the key growth factors. However, pricing pressure due to the availability of generics and biosimilars in the region may hamper the market revenue to some extent in the coming years. Overall, increased healthcare investment, expanding patient pool and growing focus on innovative treatment options will aid the Europe incretin mimetics market during the forecast period.
Market Report Scope
Incretin Mimetics Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 23.84 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.0% | 2032 Value Projection: | USD 33.56 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GSK, Novo Nordisk, Lily, Haosoh, Sanofi, AstraZeneca, Pfizer Inc, Bristol-Myers Squibb Company, Teva Pharmaceutical Industries Ltd, Sun Pharmaceutical Industries Ltd, Daiichi Sankyo Company, Limited, AbbVie Inc., Dr. Reddy’s Laboratories Ltd., Torrent Pharmaceuticals Ltd., Glenmark Pharmaceuticals Ltd., Cadila Healthcare Ltd., Alembic Pharmaceuticals Limited , Boehringer Ingelheim |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Incretin Mimetics Industry News
- In January 2024, Glenmark Pharmaceuticals Ltd., a research-driven global pharmaceutical business, launched a biosimilar of the popular anti-diabetic medicine Liraglutide for the first time in India. The medicine is being marketed under the brand name Lirafit after receiving permission from the medicine Controller General of India (DCGI).
- In August 2023, Dr. Reddy's Laboratories Ltd., a global pharmaceutical company, announced the launch of Saxagliptin and Metformin Hydrochloride Extended-Release Tablets in the U.S. These tablets are a therapeutic equivalent generic version of KOMBIGLYZE XR (saxagliptin and metformin hydrochloride extended release) tablets that have been approved by the U.S. Food and Drug Administration.
- In November 2022, Biocon Limited, an innovation-driven global biopharmaceutical company, signed a semi-exclusive partnership agreement with Zentiva, a leading European pharmaceutical company, to commercialize Liraglutide, a vertically integrated, complex formulation for the treatment and management of Type 2 diabetes and obesity.
- In July 2021, AstraZeneca, a multinational biotechnology company, announced that its product BYDUREON BCise (exenatide extended-release), a once-weekly injectable suspension, had been approved in the U.S. by US FDA for the treatment of type 2 diabetes (T2D), to improve glycemic control in pediatric patients aged 10 to 17 years as an adjunct to diet and exercise.
*Definition: The incretin mimetics market consists of pharmaceutical therapies that mimic the effects of incretin hormones like glucagon-like peptide-1 (GLP-1). These medications are used to treat patients with type 2 diabetes by stimulating the secretion of insulin from pancreatic beta cells. They work by binding to and activating the GLP-1 receptor, helping to regulate blood glucose levels while reducing the risk of hypoglycemia. Common brand names of incretin mimetic drugs include Victoza, Byetta, and other.
Market Segmentation
- Drug Type Insights (Revenue, USD BN, 2020 - 2032)
- Exenatide
- Liraglutide
- Sitagliptin
- Saxagliptin
- Alogliptin
- Others
- Disease Indication Insights (Revenue, USD BN, 2020 - 2032)
- Type 2 Diabetes Mellitus (T2DM)
- Weight Management
- Others
- Distribution Channel Insights (Revenue, USD BN, 2020 - 2032)
- Online Pharmacies
- Retail Pharmacies
- Hospital Pharmacies
- Regional Insights (Revenue, USD BN, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- GSK
- Novo Nordisk
- Lily
- Haosoh
- Sanofi
- AstraZeneca
- Pfizer Inc
- Bristol-Myers Squibb Company
- Teva Pharmaceutical Industries Ltd
- Sun Pharmaceutical Industries Ltd
- Daiichi Sankyo Company, Limited
- AbbVie Inc.
- Dr. Reddy’s Laboratories Ltd.
- Torrent Pharmaceuticals Ltd.
- Glenmark Pharmaceuticals Ltd.
- Cadila Healthcare Ltd.
- Alembic Pharmaceuticals Limited
- Boehringer Ingelheim
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
