In Vivo CRO Market Size and Trends
The global in vivo CRO market is estimated to be valued at USD 5.11 Bn in 2025 and is expected to reach USD 9.17 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 8.7% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The global in vivo CRO market has been witnessing significant growth over the past few years. Rising investments in R&D activities by biopharmaceutical companies and increasing outsourcing of preclinical studies are driving the market growth. Moreover, the growing complexity of diseases and development of novel molecules has raised the need for efficient preclinical testing. CROs, Contract research organization provide end-to-end services for preclinical studies which help pharmaceutical companies focus on their core areas. Furthermore, capabilities of CROs to conduct preclinical studies within stipulated timelines and budgets while maintaining high quality standards is also fueling their adoption rate. However, high costs associated with preclinical animal research may hamper the market growth to some extent over the forecast period.
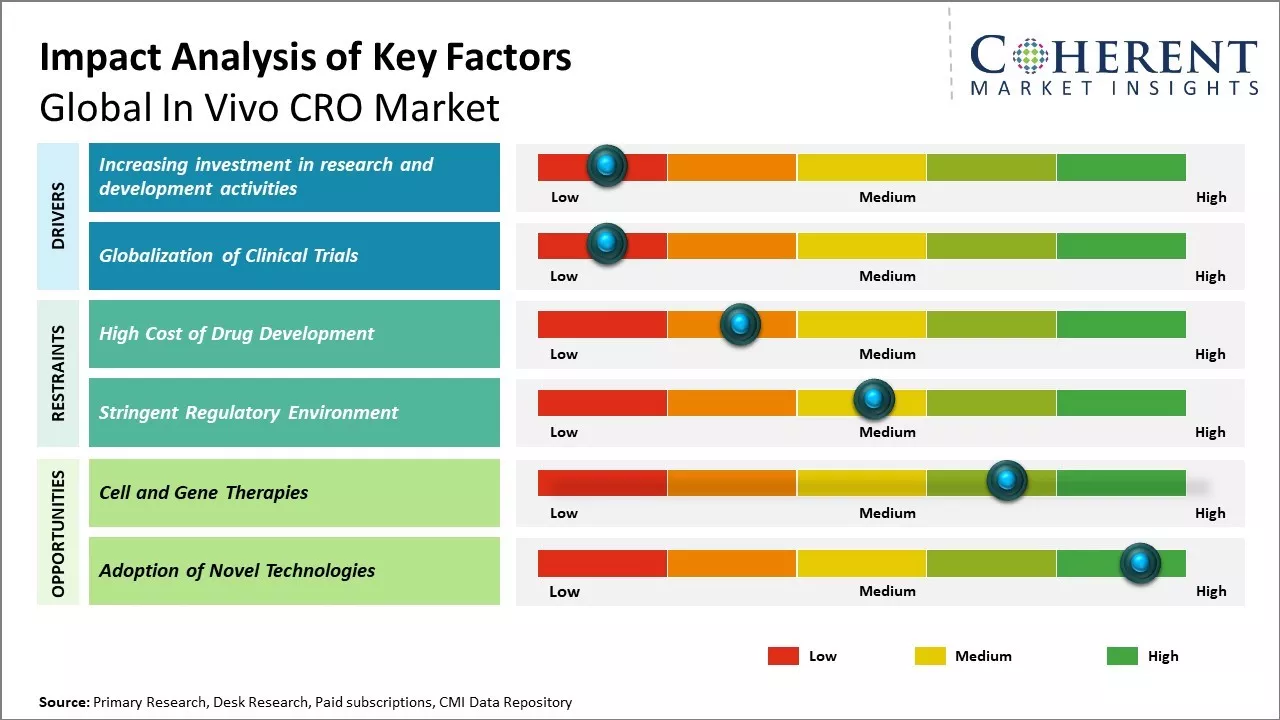
Increasing investment in research and development activities
Increasing research activities devoted towards the advancement of healthcare is positively impacting the growth of the global in vivo contract research organization market. Pharmaceutical and biotechnology companies are augmenting their investments in R&D to develop novel therapies and treatment options for chronic and life-threatening diseases. This has led to the rising demand for outsourcing preclinical research studies to specialty contract research organizations. CROs with in vivo facilities offer tailor-made research services spanning across discovery, preclinical, and translational stages of drug development. Their core competencies include disease modeling, target validation, efficacy, and safety testing using animals and advanced analytical techniques. Outsourcing to expert CROs allows drug developers to stay agile while focusing on internal resources on later phase trials and commercialization. It also helps them mitigate regulatory risks and cost burdens associated with developing in-house research infrastructure and hiring specialized workforce. For instance, in November 2021, Merck & Co. Inc., a multinational pharmaceutical company, finalized an agreement to purchase Velosbio Inc., a privately-held clinical-stage biopharmaceutical firm focused on pioneering cancer treatments aimed at receptor tyrosine kinase-like orphan receptor 1 (ROR1). Likewise, in March 2021, Johnson & Johnson, a global pharmaceutical company, was granted Breakthrough Therapy Designation by the U.S. FDA for JNJ-61186372 (JNJ-6372) for treating patients with metastatic non-small cell lung cancer (NSCLC).
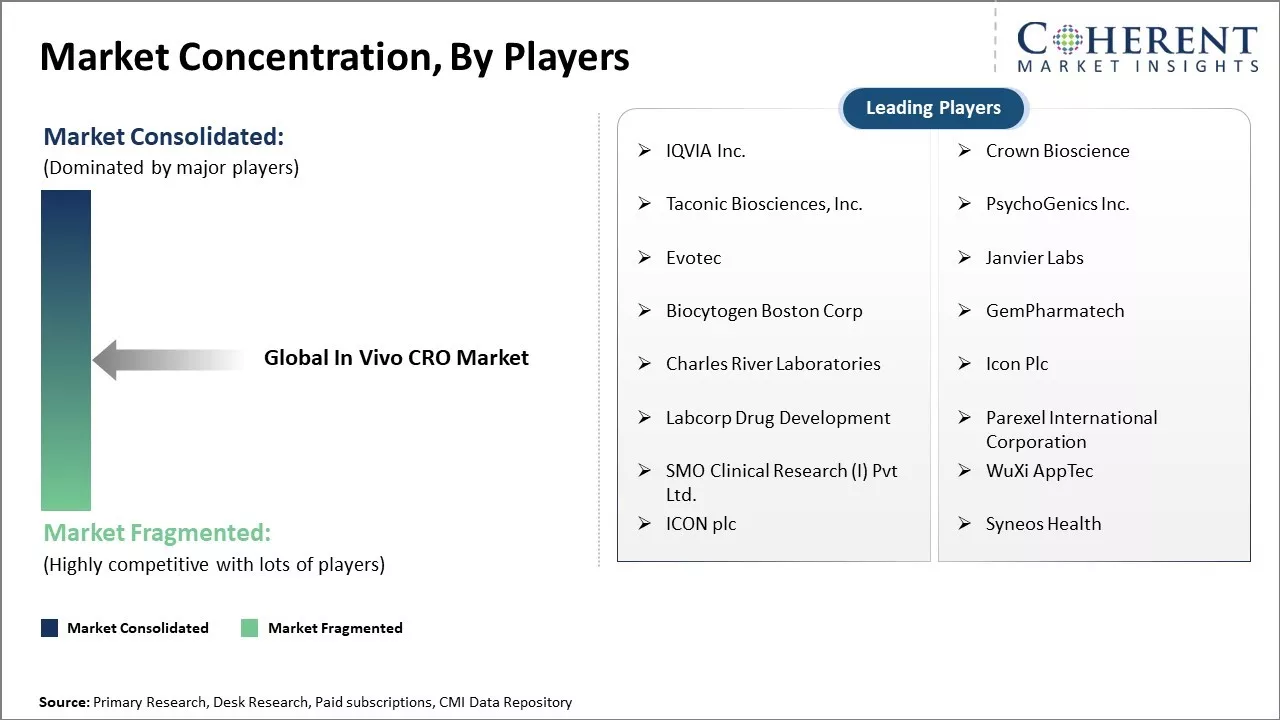
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Globalization of Clinical Trials
With growing patient populations and disease burdens in emerging markets, drug developers are seeking to enroll more trial subjects and complete studies faster. This has led to the rapid globalization of clinical trial landscape over the past decade. Companies now conduct a significant portion of their clinical trials outside traditional hubs in the U.S. and Europe. Countries across Asia, Latin America, Eastern Europe, and Africa now contribute sizeable shares to global trials. For e.g., over 60% of trials in 2020 recruited at least one subject outside U.S. and Europe combined. This trend is mostly driven by advantages like the availability of treatment naïve patients, lower costs, and quicker site initiation in these regions. However, conducting trials internationally also poses several operational and regulatory challenges. Variations exist in local ethical and biosafety norms, documentation requirements, and standards of care. Sponsors require experienced partners to navigate these complexities smoothly across global sites.
Key Takeaways from Analyst
The global in vivo CRO market is expected to see steady growth over the next five years. Some key drivers of this market include the increasing R&D expenditure of pharmaceutical companies and growing demand for outsourcing preclinical studies. Many big pharmaceutical companies are shifting their focus towards specialty areas and outsourcing non-core activities like preclinical research to CROs. This trend is expected to continue supplementing the market growth.
However, stringent regulations surrounding animal testing and rising concerns over animal welfare rights are likely to pose challenges. Sponsors may also experience delays in timelines if CROs face regulatory issues. Retaining trained professionals is another restraint as there is a high demand-supply gap for specialized skills.
North America will likely maintain its dominance owing to the concentration of big pharmaceutical companies. Meanwhile, Asia Pacific offers lucrative opportunities and is expected to witness the fastest gains. Many sponsors are shifting their focus to this region given advantages like lower operational costs and a large talent pool. China and India, in particular, are emerging as popular preclinical outsourcing destinations.
Market Challenges: High Cost of Drug Development
The high cost of drug development is significantly restraining the growth of the global in vivo CRO market. Developing new drug therapies is an extremely expensive process, with the average cost estimated to be over US$ 2.60 billion according to a 2020 report by the Tufts Center for the Study of Drug Development. This includes everything from conducting basic research and clinical trials to getting regulatory approval. Given these enormous costs involved, pharmaceutical companies are under extreme pressure to maximize their returns on investment for new drugs. Any aspect of the development process that adds to the costs or risks is viewed very unfavorably by big pharmaceutical sponsors. Outsourcing studies to contract research organizations (CROs) is seen as adding an additional layer of expenses and reducing the sponsors' control over their proprietary compounds and data. While CROs offer expertise and scale that pharmaceutical companies lack, their involvement increases overall project budgets and timelines. This makes sponsors wary of relying too heavily on CROs, preferring to handle more of the work in-house instead.
Market Opportunities: Cell and Gene Therapies
Cell and gene therapies could open up a huge opportunity for the global in vivo CRO market in this decade. These novel therapies are revolutionizing treatment approaches by addressing the underlying cause of diseases rather than just alleviating symptoms. This paradigm shift from managing to curing serious diseases has massive future growth potential. Several drug developers are actively conducting clinical trials of cell and gene therapies across therapeutic areas like oncology, neurological disorders, genetic disorders, etc. For example, the FDA approved the first gene therapy treatment for spinal muscular atrophy in 2020 (National Institute of Health). Many others are in late-stage trials. As these novel treatment modalities receive approvals and rollout at commercial scale, the need for specialized testing services by in vivo CROs will exponentially increase in areas such as preclinical research, toxicology studies, biodistribution studies, delivery mechanism evaluation, etc. This represents a major transition underway in drug development, triggered by the promise of one-time curative interventions for conditions considered incurable so far. In vivo CRO companies will have to make large investments to build expertise specific to cell and gene therapies over the next 5 years to cater to this increased customer demand. Stem cell therapy development is another fast-emerging area with potential for regenerative medicines and management of chronic diseases. The World Health Organization (WHO) estimates that over 150 stem cell therapy trials are underway globally for a variety of conditions including COVID-19.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
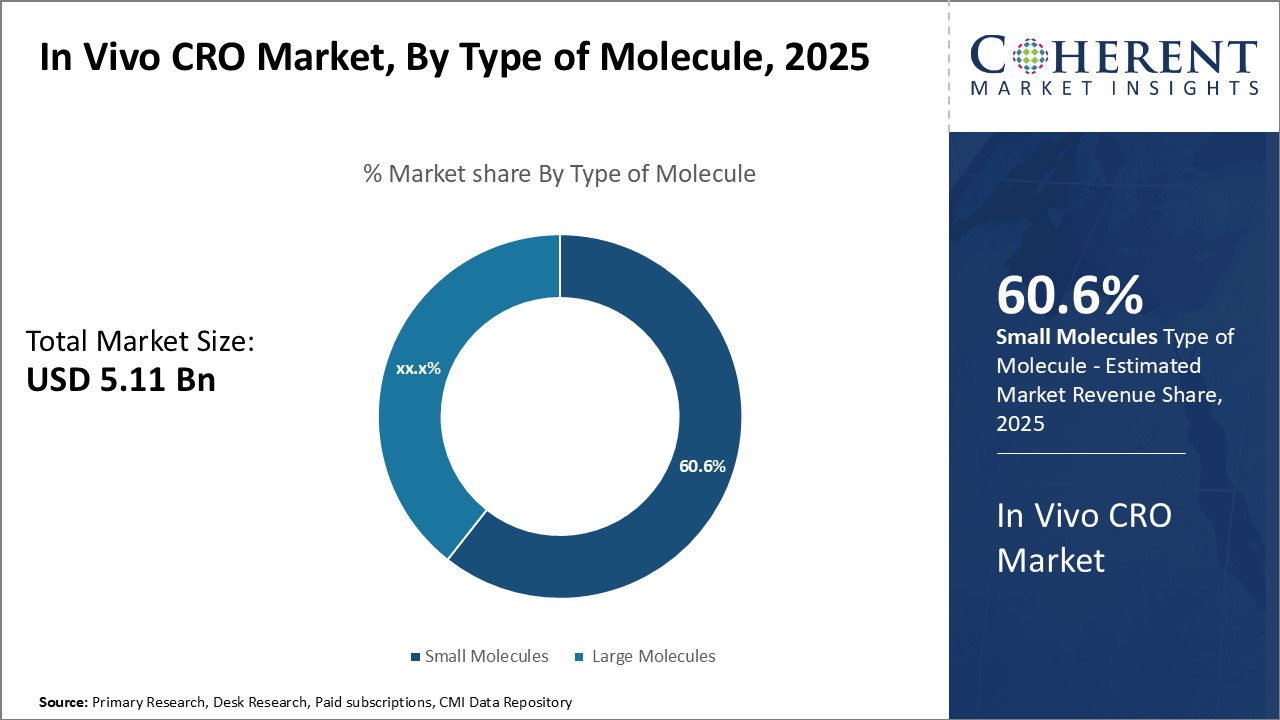
By Type of Molecule - High Demand for Cost-effective Drugs Drives Small Molecules Segment
In terms of type of molecule, small molecules is expected to contribute the highest share of the market with 60.6% in 2025 owing to their cost advantages over large molecules. Being relatively simple in structure, small molecules require less extensive manufacturing processes as compared to biologics and biosimilars. This makes them significantly more affordable for patients. Their ease of production also translates to lower costs for pharmaceutical companies. As a result, small molecules remain the mainstay for treatment of many chronic diseases and conditions that affect a large patient population worldwide. The lower costs allow for more widespread access and adoption of novel small molecule drugs. Additionally, many small molecules have well-established mechanisms of action and safely profiles based on decades of clinical use. This gives confidence to drug developers seeking new therapies. As the trend of value-based healthcare intensifies globally, the demand for low-cost yet effective treatment options like small molecule drugs will continue driving this segment.
By Therapeutic Area - Oncology Leads Due to Growing Cancer Burden and Research Prioritization
In terms of therapeutic area, oncology is expected to contribute the highest share of the market, accounting for 25.5% pf the market share in 2025. This can be attributed to continuous increasing incidence and prevalence of various cancer types worldwide. Cancer poses a huge medical and economic challenge with its lack of effective cures for many forms. Consequently, oncology attracts significant research funding and industry investment towards the development of advanced diagnostic tools and newer therapeutic drugs and regimens. A vast amount of pre-clinical and clinical data is continuously generated from cancer research projects. This dependence on in vivo studies to evaluate new molecules for oncology indications ensures a steady flow of activities and revenue for CROs specializing in this area. Additionally, innovations in precision oncology have opened up new avenues for personalized and targeted therapies development, further fueling research volumes. Given the urgency to find new cancer solutions and treatments, oncology will remain the foremost driver of in vivo CRO market.
By Service Type - Preclinical Testing Holds Dominance Due to the Importance of Early-stage Evaluation
In terms of service type, preclinical testing is expected to contribute the highest share of the market with 40.6% in 2025 owing to its status as an indispensable early phase in the drug development process. Before clinical trials in humans, it is mandatory to investigate drug pharmacology and toxicity using relevant preclinical disease models and animal subjects. This provides crucial insights regarding drug metabolism, pharmacokinetics and safety profile prior to first-in-human studies. As the importance of predictive toxicology increases due to regulatory pressures, sponsors look to outsource preclinical testing to specialized CROs having required expertise and infrastructure. Furthermore, strict guidelines necessitate sophisticated preclinical programs spanning multiple animal species to identify and mitigate risks early on. Therefore, CROs offering diverse preclinical services play a pivotal role in assisting biopharma clients meet statutory requirements for clinical trials approval. Their involvement secures a steady flow of preclinical test orders, cementing this segment's lead.
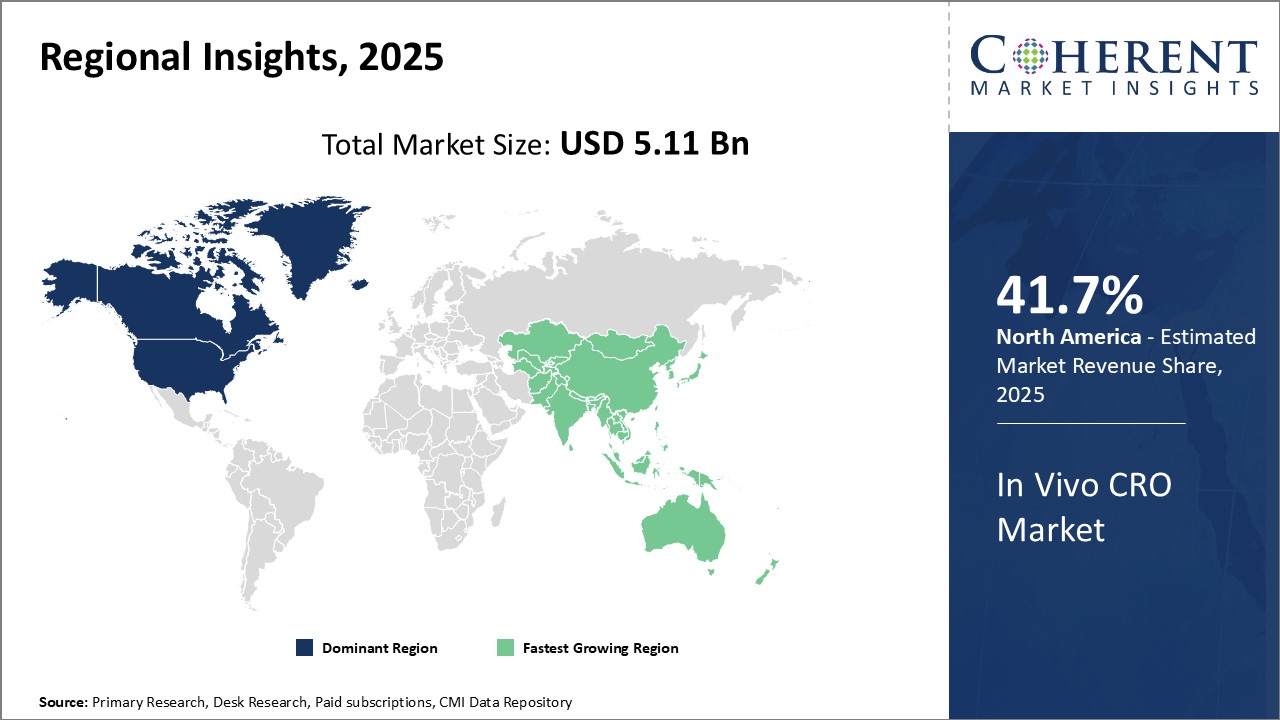
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America has dominated the global in vivo CRO market for several years. The region is expected to account for 41.7% of the market share in 2025. This region is home to several top pharmaceutical companies who outsource a major portion of their preclinical research activities. With a strong presence of CROs catering to the life sciences industry and high investments in R&D, North America provides ideal conditions for growth of this market. The region also has stringent regulations for product testing and development which drives demand for specialized preclinical services. Several global players have established their headquarters and major facilities in the U.S. and Canada to leverage the skilled workforce and expertise available. This has ensured that North America remains the primary export and import hub for in vivo CRO services internationally.
The Asia Pacific region is poised to be the fastest growing market in the coming years. Countries like China, India, and South Korea offer lower operational costs compared to mature markets, attracting several pharmaceutical giants to outsource their preclinical work to Asia Pacific. This has encouraged local and international CROs to expand their footprint in the region to capture a share of the outsourced business. The economic development of Asia Pacific countries has also led to greater healthcare investments and a focus on developing domestic life sciences capabilities. This is increasing the demand for specialized in vivo services to support drug development programs within the region. With growing expertise, a large talent pool and proximity to resource-rich nations, Asia Pacific is well positioned to take over as the dominant outsourcing destination for global in vivo CRO requirements.
Market Report Scope
In Vivo CRO Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 5.11 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.7% | 2032 Value Projection: | USD 9.17 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
IQVIA Inc., Crown Bioscience, Taconic Biosciences, Inc., PsychoGenics Inc., Evotec, Janvier Labs, Biocytogen Boston Corp, GemPharmatech, Charles River Laboratories, Icon Plc, Labcorp Drug Development, Parexel International Corporation, SMO Clinical Research (I) Pvt Ltd., WuXi AppTec, ICON plc, and Syneos Health |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
In Vivo CRO Industry News
- In March 2022, eTheRN, a biotechnology company launched a new service for developing and producing Lipid Nanoparticle (LNP) formulations. This service aims to assist in the discovery and early pre-clinical development of RNA-based therapeutics and vaccines.
- In November 2023, Charles River Laboratories, a U.S.-based pharmaceutical company, revealed a collaboration with Aitia, biosimulation compan focused on drug development and in vivo oncology research
- In March 2023, Biocytogen Boston Corp, a pharmaceutical company, announced a licensing agreement with Janssen Biotech, Inc., granting Janssen Biotech, Inc. rights to utilize the RenLite platform
- In January 2023, Evotec SE, a drug discovery and development company, forged a partnership with Janssen Biotech, a biotech company, to advance immune-based therapies targeting oncology
*Definition: The in vivo CRO market consists of pharmaceutical drugs that are used to treat kidney cancer. The market includes drugs from different drug classes that are used to target different molecular pathways involved in the development and progression of kidney cancers. Some of the major drug classes included in this market are targeted therapies such as tyrosine kinase inhibitors, mTOR inhibitors, angiogenesis inhibitors; immunotherapies such as checkpoint inhibitors; and chemotherapies.
Market Segmentation
- Type of Molecule Insights (Revenue, USD Bn, 2020 - 2032)
- Small Molecules
- Large Molecules
- Therapeutic Area Insights (Revenue, USD Bn, 2020 - 2032)
- Oncology
- Cardiology
- Diabetes
- Autoimmune/inflammation conditions
- Infectious Diseases
- CNS Conditions
- Others
- Service Type Insights (Revenue, USD Bn, 2020 - 2032)
- Preclinical Testing
- Clinical Research Services
- Laboratory Services
- Consulting Services
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- IQVIA Inc.
- Crown Bioscience
- Taconic Biosciences, Inc.
- PsychoGenics Inc.
- Evotec
- Janvier Labs
- Biocytogen Boston Corp
- GemPharmatech
- Charles River Laboratories
- Icon Plc
- Labcorp Drug Development
- Parexel International Corporation
- SMO Clinical Research (I) Pvt Ltd.
- WuXi AppTec
- ICON plc
- Syneos Health
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
