HIV Clinical Trials Market Size and Trends
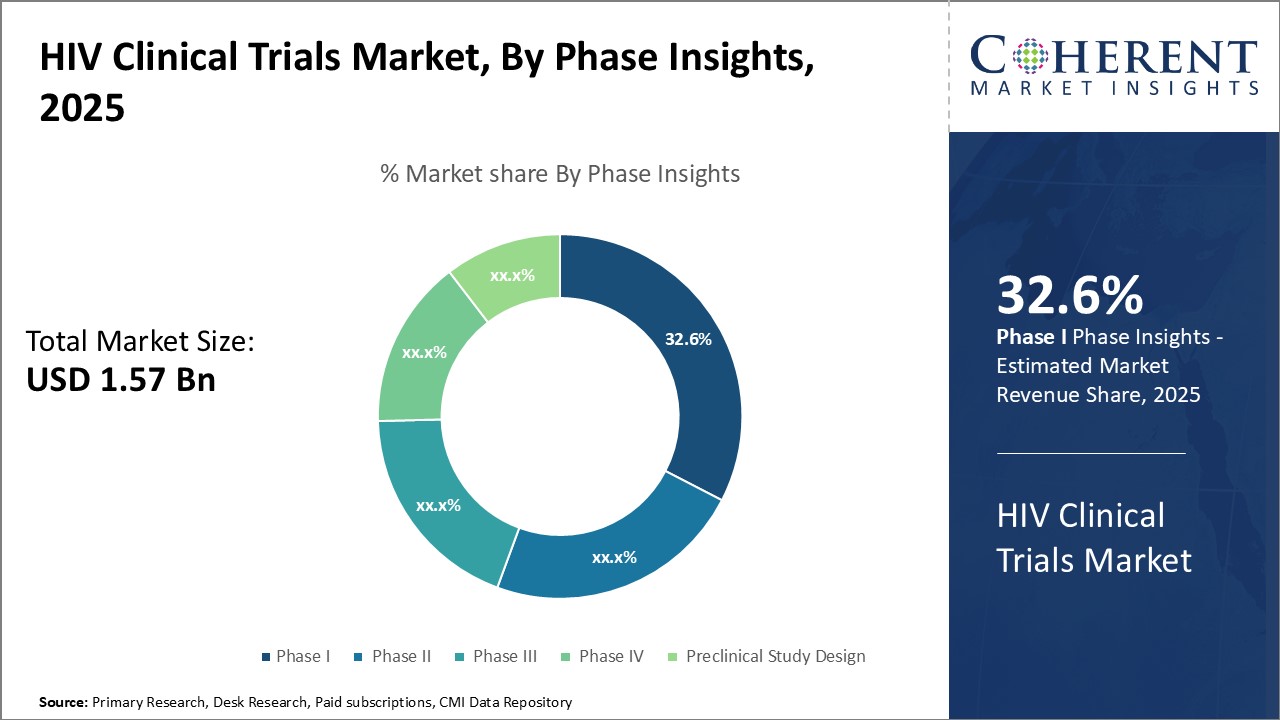
Global HIV clinical trials market is estimated to be valued at USD 1.57 Bn in 2025 and is expected to reach USD 2.36 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.0% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
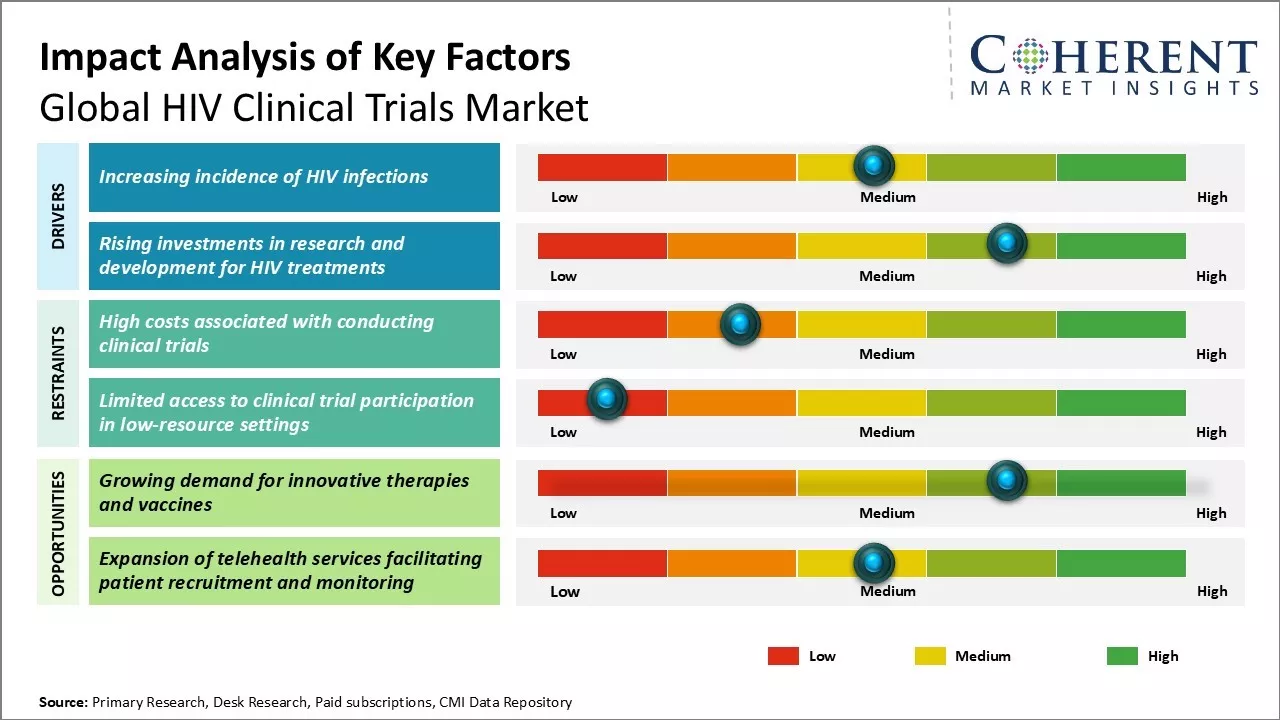
The market can witness positive growth during the forecast period due to factors such as rising number of people suffering from HIV/AIDS globally, increasing R&D investment in drug development by key pharmaceutical players, and growing government support for conducting clinical trials. Moreover, increasing demand for effective and highly innovative drugs for treatment of HIV/AIDS can also drive the market growth in the near future. However, high costs associated with clinical trial material and lengthy U.S. FDA approval process could hamper the market growth during the forecast period.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
Insights, By Phase- Clinical advancement drives phase I contributions
In terms of phase, phase I segment is estimated to contribute the highest share of the HIV clinical trials market with 32.6% in 2025, owing to its critical role in testing experimental treatments. Phase I trials lay the groundwork for subsequent research by evaluating a new drug's safety, determining a safe dosage range, and identifying possible side effects. Given the exploratory nature of Phase I, a high level of uncertainty exists around both clinical efficacy and commercial viability.
Insights, By Study Design - Interventional research shapes Interventional dominance
In terms of study design, the interventional studies segment is estimated to contribute the highest market share of 43.6% in 2025, owing to their ability to directly assess clinical effectiveness. Interventional trials test the impact of an experimental treatment compared to standard care or placebo in a prospective, controlled manner. This provides robust evidence for regulatory approval and physician adoption. However, their expansive scope requires sizable resources. Interventional studies take advantage of existing subject pools and real-world settings to generate complementary insights with lower costs.
Insights, By Sponsor Type - Academic research drives government involvement
In terms of sponsor type, the pharmaceutical & biotechnology companies segment dominates the global HIV clinical trials market with 41.8% in 2025 due to their significant investment in the development of HIV treatments, including antiretroviral therapies and vaccines. These companies have the largest financial resources, enabling them to conduct large-scale trials. Their focus on innovation and market demand for effective HIV solutions further drives their leadership.
Regional Insights
Need a Different Region or Segment? Download Free Sample
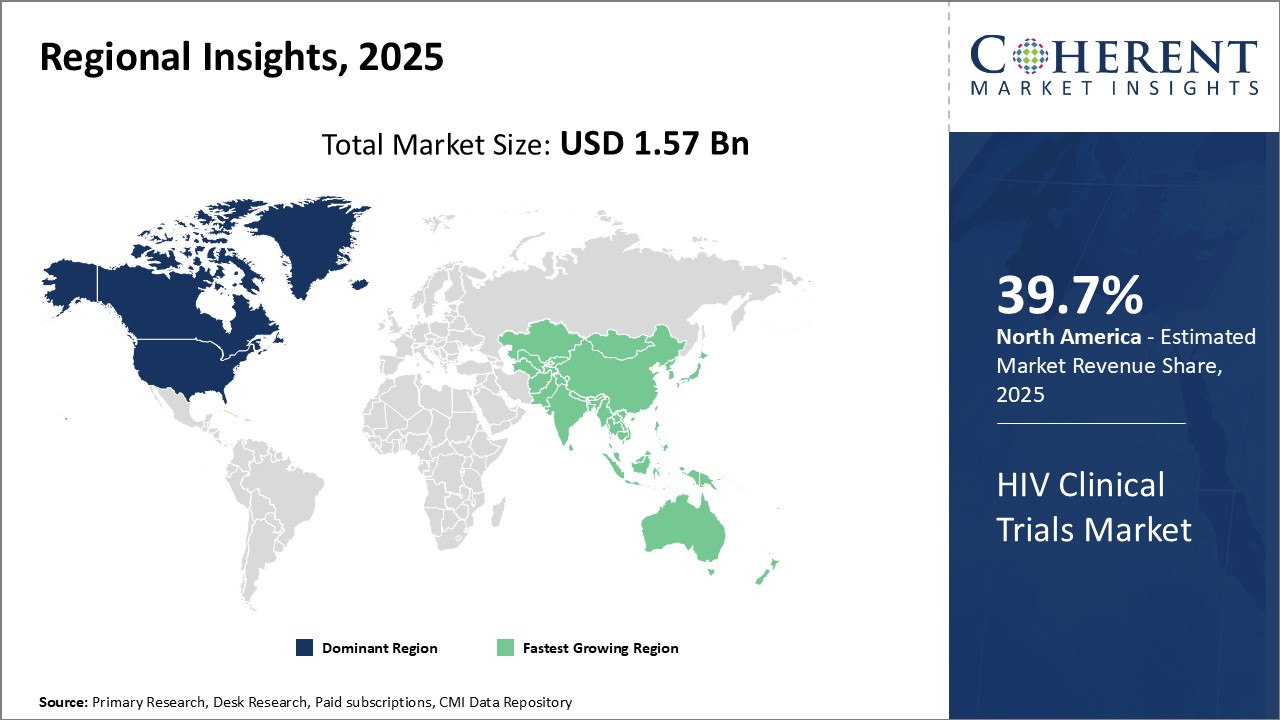
Dominating Region- North America
North America dominates the HIV clinical trials market with an estimated market share of 39.7% in 2025, due to presence of major pharmaceutical companies like AbbVie Inc. and Johnson & Johnson and contract research organizations (CROs) in the region. The region also has a supportive regulatory environment and high healthcare expenditure which promotes clinical research activities.
Fastest-Growing Region- Asia Pacific
Asia Pacific region exhibits the fastest growth and is emerging as a key market for HIV clinical trials. This can be attributed to rising investment by international players to leverage low-cost opportunities and growing patient population in countries like China and India. Regional governments are also encouraging clinical research to improve medical capabilities.
HIV Clinical Trials Market Outlook for Key Countries
U.S.- Leading Hub for HIV Clinical Trials and Innovation
The U.S. HIV clinical trials market leads due to top sponsors and trial sites. For instance, in September 2023, a Phase 1 trial for the novel HIV vaccine VIR-1388 began in the U.S. and South Africa. Funded by the National Institute of Allergy and Infectious Diseases (NIAID) and sponsored by Vir Biotechnology, the trial aims to test the safety and immune response of the vaccine, enrolling 95 HIV-negative participants.
Canada- Growing Healthcare Infrastructure Drives HIV Clinical Trials
The HIV clinical trials market in Canada is driven by increasing number of clinical trials and sites, along with the country’s robust healthcare infrastructure for conducting these trials. Furthermore, ongoing research and development, product innovation, and approvals are anticipated to boost the market growth. For instance, in April 2022, according to the government of Canada, approximately 77.5% of biopharmaceutical products are in the early stages of research and development (from research to phase I/II) In Canada, while 23.5% are in the mid- to late-stage of development (late-stage clinical trials). These government initiatives are expected to drive market expansion.
India- Cost-Effective and Diverse Patient Pool Boosting HIV Clinical Trials
The HIV clinical trials market in India is fueled by cost-effectiveness, a diverse patient population that makes it an appealing location for clinical trials, and growing research activities by organizations. For instance, in October 2023, Roche Pharma launched the Clinical Trial Excellence Project in India. The project aims to enhance the capabilities of public health institutions to conduct clinical trials and drug research. It will transform government hospitals into centers of excellence for clinical research, further, boosting the market.
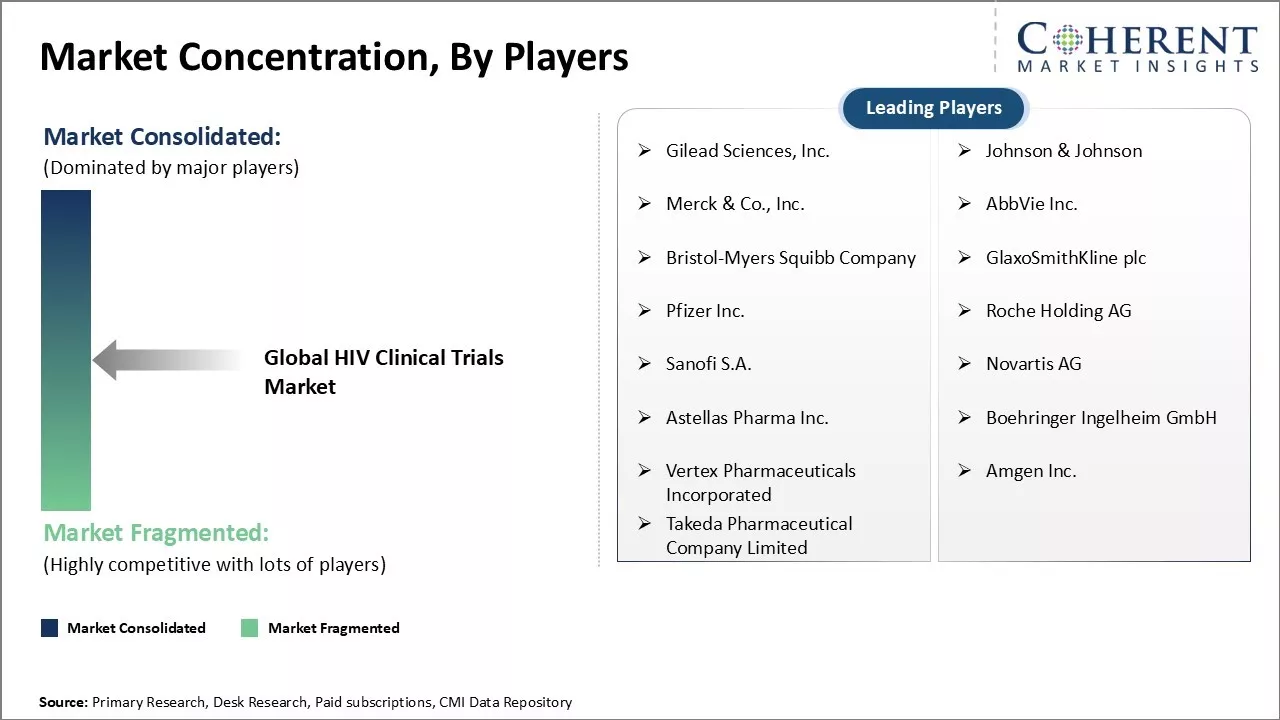
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Global HIV Clinical Trials Market Players
- Established Players- Companies are focusing on novel therapies to enhance patient outcomes. For instance, Gilead Sciences spends over 20% of its annual revenues on R&D activities focused on developing new drug formulations and testing trials. Its recent pipeline includes long-acting therapies that only need to be administered monthly.
- Mid-Level Players- Mid-sized companies in the HIV clinical trials market focus on delivering high-quality and affordable treatment solutions. For example, Merck Sharp & Dohme invests in robust manufacturing infrastructure in low-cost regions. This helps reduce production expenses and enables them to price drugs competitively. Boehringer Ingelheim runs clinical trials in developing nations where patient recruitment and site costs are lower.
- Small-Scale Players- New entrants often target specific segments left untapped by larger firms. Anthropic focuses only on pediatric It engineers AI-powered chatbots educate children about self-management of the condition. Altoida Biosciences concentrates on developing user-friendly rapid testing kits ideal for point-of-care diagnosis in remote areas.
Emerging Startups in the Global HIV Clinical Trials Market
Innovative Technologies- Recent startups are introducing innovative technologies, such as Dexcom, which is developing implantable continuous glucose monitors integrated with AI. These sensors help optimize drug dosages in patients by providing real-time blood monitoring to prevent virus mutations. Additionally, SynSense is creating wireless smart patches that non-invasively track vital signs, monitor medication adherence, and alert healthcare providers to potential health deteriorations.
Sustainable Solutions- Startups like TerraCycle focus on sustainability by converting clinical waste like used syringes, vials, and others into reusable plastics using unique recycling technologies. Its solutions help reduce environmental pollution from medical kits. Poett manufactures medicines using plant-based packaging that can decompose without harming the ecosystem.
Key Takeaways from Analyst
- Global HIV clinical trials market growth is driven by increasing prevalence of HIV/AIDS worldwide. The market growth is driven by rising investments from governments and non-profit organizations to develop improved treatment options for HIV patients. Growing demand for newer drug formulations such as long-acting antiretrovirals which require less frequent dosing can support market expansion.
- Stringent regulations for drug approval and long approval timelines pose challenges for growth of HIV clinical trials. Conducting international multi-center trials also involve substantial investments pertaining to participant recruitment and retention.
- North America dominates the market due to presence of majority of global pharmaceutical vendors and clinical research organizations. However, Asia Pacific is likely to gain significant market share, owing to large patient pools and lower costs for clinical trials. Improving healthcare infrastructure and regulatory framework in many Asian countries attracts several pharmaceutical companies to conduct late phase trials.
- Ongoing research for HIV cure and vaccine presents tremendous opportunities for market players. Partnerships between drug makers, research institutes and non-profit organizations should help in faster clinical development. Furthermore, growing emergence of biosimilars will augment the clinical trial testing needs in the future.
Market Report Scope
HIV Clinical Trials Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.57 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.0% | 2032 Value Projection: | USD 2.36 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Gilead Sciences, Inc., Johnson & Johnson, Merck & Co., Inc., AbbVie Inc., Bristol-Myers Squibb Company, GlaxoSmithKline plc, Pfizer Inc., Roche Holding AG, Sanofi S.A., Novartis AG, Astellas Pharma Inc., Boehringer Ingelheim GmbH, Vertex Pharmaceuticals Incorporated, Amgen Inc., and Takeda Pharmaceutical Company Limited |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Dynamics
Market Driver- Increasing incidence of HIV infections
The prevalence of HIV infections has increased in recent years. According to WHO data published in July 2024, by 2023, approximately 39.9 million people, including 1.4 million children, were living with HIV. In 2023, there were 1.3 million new HIV infections, reflecting a 39% reduction since 2010. HIV-related deaths also decreased by 51% since 2010, with 630,000 deaths reported in 2023. By 2023, 86% of people living with HIV were aware of their status, leaving 3.4 million individuals still to be tested.
Market Challenge- High costs associated with conducting clinical trials
Conducting clinical trials for HIV therapies and vaccines is an extremely costly process. Various phases of clinical trials require recruiting thousands of human participants and carrying out complex research studies over long periods of time. Moreover, standard therapies and diagnostics need to undergo rigorous testing to prove their safety and efficacy as per regulatory guidelines. This involves setting up proper clinical infrastructure at trial sites, hiring and training clinical research personnel, developing investigational drugs and products, monitoring trials and collecting and analyzing vast amounts of clinical data.
Market Opportunity- Growing demand for innovative therapies and vaccines
There has been growing global demand for more effective, safer and affordable innovative therapies and vaccines for the treatment and prevention of HIV/AIDS. While current antiretroviral treatments have increased lifespan of HIV patients, these regimens still face issues of drug resistance, toxicities, requirement of lifelong adherence and socioeconomic inaccessibility in developing countries. No vaccine has been proven to completely prevent HIV infection even after decades of research.
Market Segmentation
- Phase Insights (Revenue, USD Bn, 2020 - 2032)
-
- Phase I
- Phase II
- Phase III
- Phase IV
- Preclinical
- Study Design Insights (Revenue, USD Bn, 2020 - 2032)
-
- Interventional Studies
- Observational Studies
- Expanded Access Studies
- Sponsor Type Insights (Revenue, USD Bn, 2020 - 2032)
-
- Pharmaceutical & Biotechnology Companies
- Academic Research Institutes
- Government & Private Research Organizations
- Contract Research Organizations (CROs)
- Indication Insights (Revenue, USD Bn, 2020 - 2032)
-
- HIV-1
- HIV-2
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
-
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Gilead Sciences, Inc.
- Johnson & Johnson
- Merck & Co., Inc.
- AbbVie Inc.
- Bristol-Myers Squibb Company
- GlaxoSmithKline plc
- Pfizer Inc.
- Roche Holding AG
- Sanofi S.A.
- Novartis AG
- Astellas Pharma Inc.
- Boehringer Ingelheim GmbH
- Vertex Pharmaceuticals Incorporated
- Amgen Inc.
- Takeda Pharmaceutical Company Limited
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
