GMP Cell Therapy Consumables Market Size and Trends
Global GMP cell therapy consumables market is estimated to be valued at USD 18.0 Mn in 2025 and is expected to reach USD 103.8 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 28.4% from 2025 to 2032. Growing cell therapy industry and increasing investments in cellular research can boost demand for GMP-compliant products needed in cell therapy manufacturing.
Discover market dynamics shaping the industry: Download Free Sample
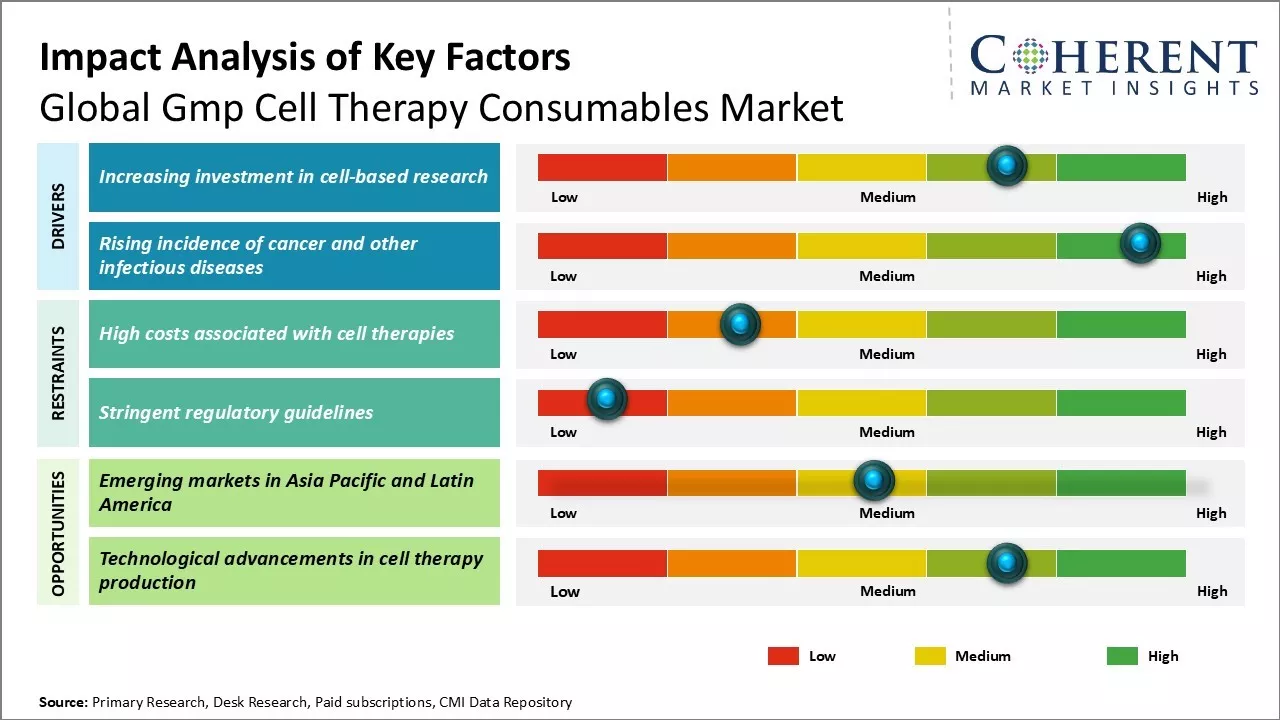
Market Driver - Increasing investment in cell-based research
Global GMP cell therapy consumables market growth is driven by rising investments directed towards cell-based research activities. Several biotech and pharmaceutical companies have increased their R&D spending on developing novel cell-based therapies as a treatment option for a wide range of medical conditions. Growth in stem cell research and regenerative medicines have also boosted investments from both private as well as public sectors.
Many market leaders in the consumables space have expanded their product portfolio as well as production facilities to cater to demands arising from cell therapy clinical trials and research projects. This is evident from rising number of collaborations between consumable suppliers and cell therapy developers. Furthermore, government initiatives aimed at providing funding and policy support for cell-based research projects have created a favorable environment for commercialization of new therapeutic applications. Regulatory approvals for advanced therapy medicinal products lead to increase in clinical research in this domain.
Research institutes and academic organizations have also increased their funding on cell-based studies related to cancer immunology, neurodegenerative disorders, genetic diseases, and others. Current pandemic situation has also boosted investments towards development of vaccine candidates based on cell culture processing. All these factors have collectively boosted demand for high-quality and GMP compliant consumables from cell therapy researchers. With greater participation from both public and private players, investments can drive the market growth.
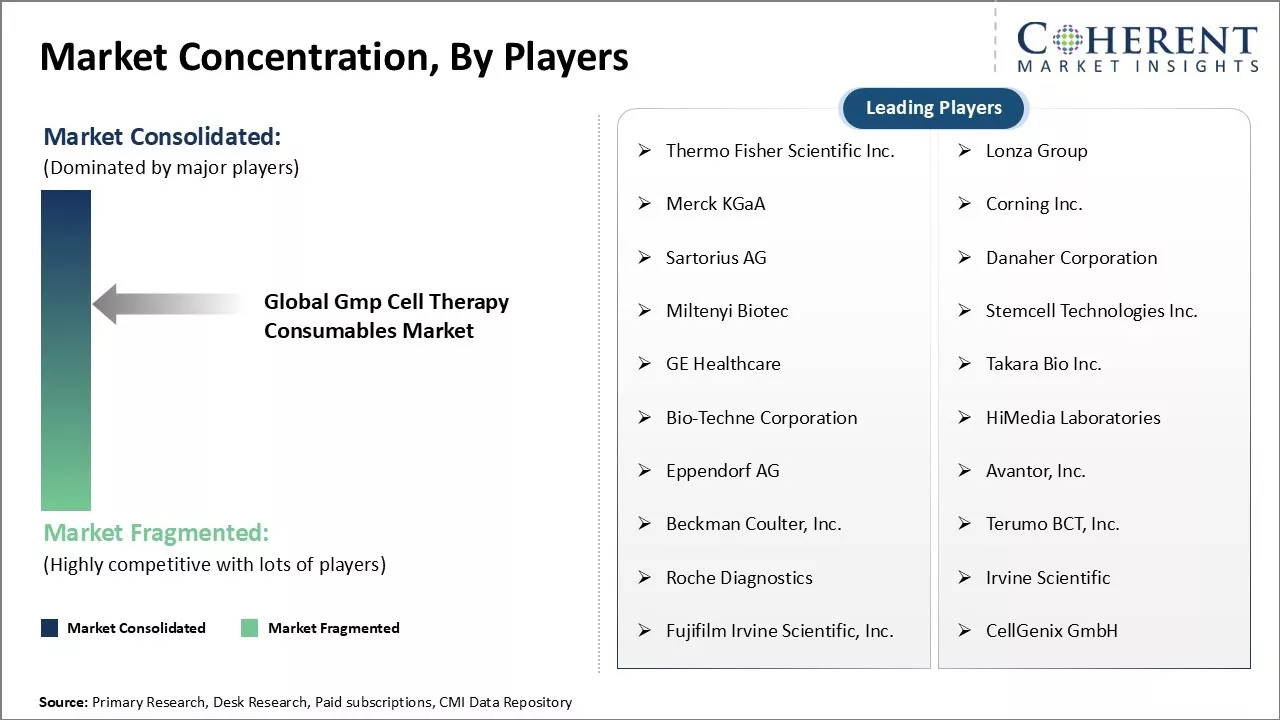
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Rising incidence of cancer and other infectious diseases
Rising prevalence of chronic medical conditions like cancer and infectious diseases can drive the global GMP cell therapy consumables market growth. According to recent statistics, cancer cases worldwide have increased in last few decades with breast, lung and colorectal cancers topping the charts. Developing nations have observed increase due to lifestyle changes and environmental factors. The economic burden of cancer on healthcare systems worldwide have escalated the need for newer treatment approaches like cell therapies.
Viral diseases such as HIV/AIDS, hepatitis, influenza, and others impact millions globally. Outbreaks of infectious illnesses including swine flu, zika virus and most recently coronavirus further highlight the urgent need for advanced therapies. Cell culture consumables have wide application from vaccine development and testing to production of therapeutic cells for these conditions. Their growth inhibiting properties, controlled environment and sterility assurance are indispensable in manufacturing cell-based products. With rising R&D for cell therapeutics, there has been huge demand for associated GMP-enabled consumables from academic as well as commercial end users.
Key Takeaways from Analyst:
Global GMP cell therapy consumables market growth is driven by advancement in cell therapy research and growing demand for regenerative medicines to treat chronic diseases. Continuous development of novel cell therapy products for different therapeutic areas can offer significant opportunities for consumable suppliers.
North America dominates the market due to presence of major players and increasing funding for cell-based research projects in the region. Asia Pacific is expected to emerge as the fastest growing market due to rising government support for cell-based research and growing biotech industry in China, Japan, and India.
High cost of cell therapy clinical trials and infrastructural challenges associated with cell processing can hamper the market growth. These restraints are likely to reduce as cell therapy technology matures. Suppliers need to focus on developing affordable consumables suitable for clinical-scale manufacturing to expand in emerging markets.
GMP cell therapy consumables market players need to closely track therapeutic advancements and changes in regulatory guidelines. Meets compliance standards will be critical for commercial success. Investments to enhance process analytical technologies and single-use solutions can help companies gain a competitive edge in this developing space.
Market Challenge - High costs associated with cell therapies
Global GMP cell therapy consumables market growth can be hampered due to high costs associated with cell therapies. Developing and manufacturing cell therapies require specialized facilities, highly trained personnel and expensive equipment. The overall production process is quite complex involving multiple steps from cell extraction, cell processing, culture media preparation to final formulation. All these steps increases the overall cost of developing a single cell therapy. Since cell therapies are personalized medicines developed for small patient pools, the manufacturing costs cannot be distributed over large volumes. The high cost of these therapies limits their access and affordability for many patients. Exorbitant costs put pressure on the healthcare systems and budgets of many countries. Unless alternative approaches are developed to reduce production costs through automation, process optimization and economies of scale, high costs will hamper the market growth.
Market Opportunity - Emerging markets in Asia Pacific and Latin America
Emerging markets in Asia Pacific and Latin America regions can offer market growth opportunities. These regions have enormous patient populations that can potentially benefit from cell therapies used for treating diseases like cancer and cardiovascular ailments. Countries like China, India, Brazil and Mexico are witnessing rapid economic growth and improvement in healthcare infrastructure. Rising affluence has increased healthcare spending capabilities in these markets. Lack of alternatives boosts adoption of advanced cell therapy approaches. To seize this opportunity, companies need to focus on developing low-cost manufacturing and delivery models that cater to these price-sensitive emerging markets. Partnering with local clinical research organizations and healthcare providers could help lower costs and boost accessibility of cell therapies in Asia Pacific and Latin American countries.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
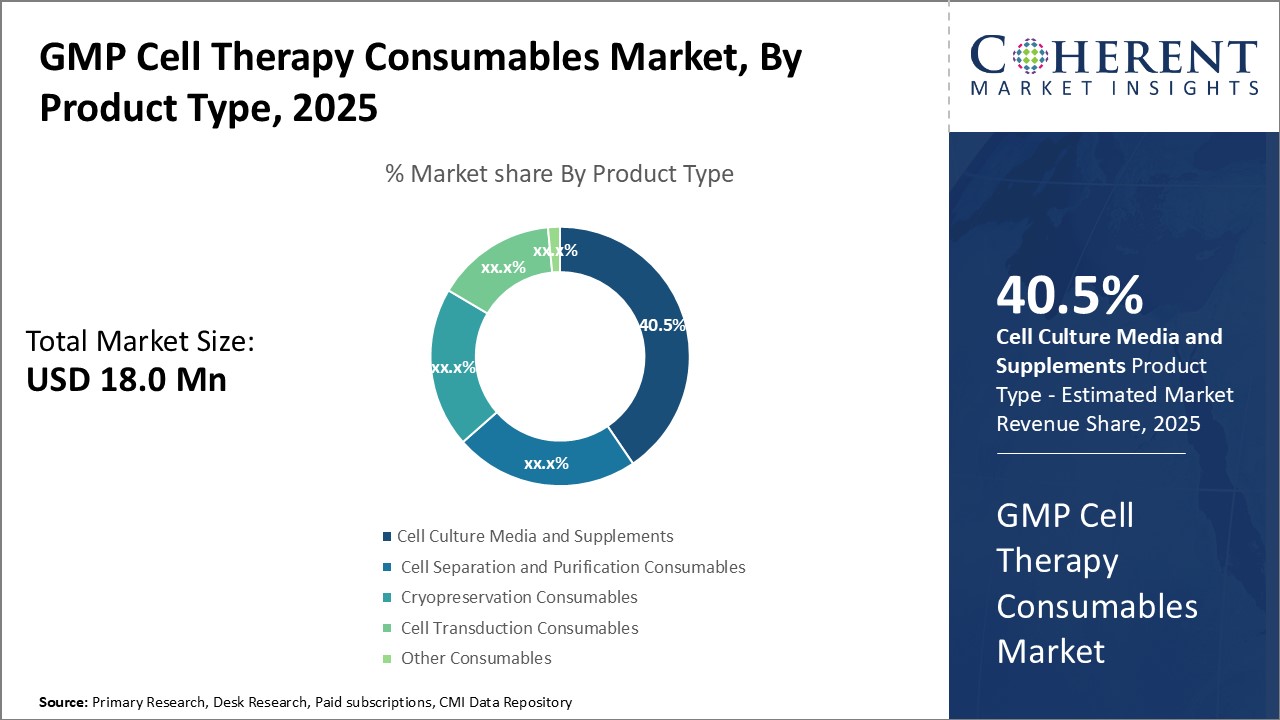
By Product Type – Rising needs of cell culture boosts demand for media and supplements
In terms of product type, cell culture media and supplements segment is estimated to contribute the highest market share of 40.5% in 2025, owning to the fundamental role these play in cell cultivation processes. Whether used for tissue engineering, regenerative medicine research, or downstream cell therapy manufacturing, all cell-based applications require specialized nutritive solutions for growing and maintaining cells outside of their natural environment in vitro. Different cell types have varying nutrient requirements that must be met through customized media formulations, in order to support proliferation, metabolism, phenotype expression and other vital functions. Due to enormous variety of cell cultures currently in use across both research and clinical settings, there has been huge demand for a wide assortment of high-quality, precisely engineered media and supplement products. Their importance has made this product segment indispensable for basic cell culture work as well as process development activities like cloning, transfection and scale-up procedures involving stem cells, adult cells and other biomaterials. Advanced products enriched with extra growth factors, cytokines and attachment substrates further aid in mimicking in vivo microenvironments and achieving robust, reproducible culture outcomes needed for translational work. Such attributes cement media and supplements as the backbone of cell-based workflows.
By Cell Type - Stem cell therapies spur growth in cell separation tools
By cell type, stem cells (embryonic, induced pluripotent, adult) segment is estimated to contribute the highest market share of 42.5% in 2025, due to expanding clinical utilization of various stem cell therapies. Mesenchymal stem cells, hematopoietic stem cells and other regenerative sources extracted from bone marrow, cord blood or adipose tissue are demonstrating promising treatment effects across a widening scope of conditions. As stem cell medicines become increasingly sophisticated and standardized, advanced cell separation and purification consumables are indispensable. Clinical-grade purity, viability and functionality are essential for ensuring the safety and efficacy profile mandated for complex stem cell therapies. Chromatographic columns, centrifugation tools, surface marker microbeads and other single-use separation consumables streamline processing while minimizing cross-contamination risks. Their ability to enrich target cell populations from heterogeneous starting materials boosts yields for lucrative downstream applications in regenerative organ reconstruction, wound healing, immune modulation and more. Automation compatible configurations also enhance scalability as stem cell therapeutic pipelines continue expanding their commercial footprint. There has been huge demand for consumables that maximize stem cell recovery, maintain multipotency and accelerate large-scale manufacturing workflows.
By Application - Oncology propels demand across therapeutic modalities
By application, oncology segment is estimated to contribute the highest market share of 43.5% in 2025, due to massive worldwide research efforts and blossoming spectrum of cell-based cancer treatments. Adoptive cell therapies leveraging engineered T cells, natural killer cells, tumor-infiltrating lymphocytes and other immunotherapies hold immense promise based on early successes against hematological and solid tumor types such as melanoma. Boosting cytotoxic activity against malignancies requires robust expansion and engineering of immune effector cells using viral transduction, electroporation, transfection and other means. This drives need for cell transduction consumables to facilitate customized reprogramming and functional enhancement of cell therapies. Beyond ACT, cancer vaccines, mesenchymal stem cell therapies, and other novel modalities are also under investigation for modulating tumor microenvironments, repairing chemotherapy damage and stimulating endogenous anti-tumor immunity. Their advancement depends on large-scale manufacturing of various cell types under pristine GMP conditions, propelling uptake of single-use bioreactor systems, pumps, tubing sets and other process consumables. Coupled with growing use of cell immunoprofiling and ex vivo drug sensitivity testing for tailoring precision oncology regimens, oncology will remain the vanguard clinical user of advanced cell therapy consumables in the future.
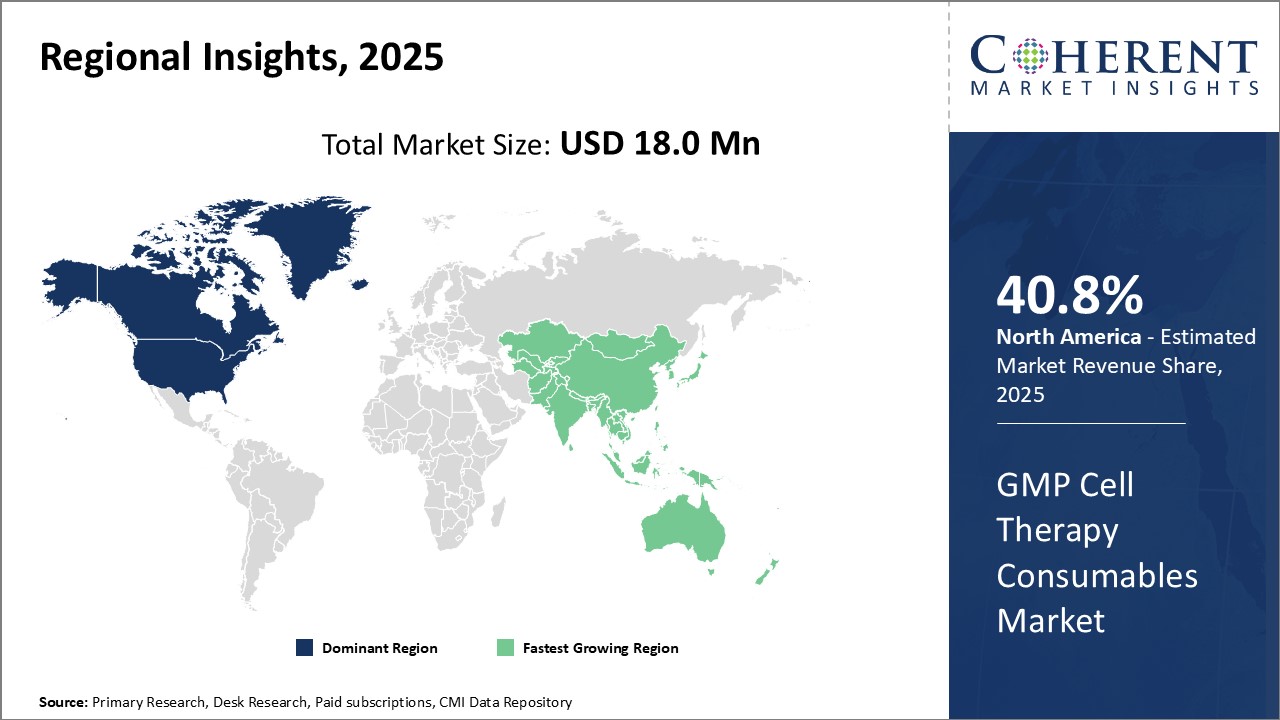
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America dominates the global GMP cell therapy consumables market with an estimated market share of 40.8% in 2025. The U.S. is home to many top pharmaceutical and biotechnology companies that are at the forefront of research and development in the cell therapy. Presence of key manufacturers in the region along with availability of skilled labor and researchers have made North America a hub for cell therapy innovations. Furthermore, supportive regulatory environment provided by U.S. FDA and increased funding for cell-based research activities have accelerated product development in this region.
Asia Pacific has emerged as the fastest growing regional market for GMP cell therapy consumables. Countries like China, Japan, South Korea and India have demonstrated high potential, owing to rising healthcare investments, increasing biopharmaceutical industry and existence of large patient pools suffering from chronic diseases. Government initiatives aimed at encouraging local manufacturing and reforms in regulatory framework are proving beneficial for both domestic as well as international players. Lower production costs and availability of low-cost raw materials have raised Asia Pacific's appeal as an attractive production base. Many western companies have set up their manufacturing units in countries like China and Singapore to cater to rising demand. Asia Pacific is projected to surpass other regions due to expanding patient pool, emerging biotech industry and increasing governmental focus on healthcare reforms in the long run.
Europe is another substantial regional market, owing to presence of major R&D focused pharmaceutical firms and supportive regulatory guidelines laid down by EMA. Countries like Germany, U.K, France are at the forefront in research activities pertaining to cell therapies. High healthcare expenditures along with universal healthcare have enabled wider access and adoption of advanced treatment procedures. However, pricing pressures from authorities and preference of biosimilars over novel therapies can hamper the market growth.
Market Report Scope
GMP Cell Therapy Consumables Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 18.0 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 28.4% | 2032 Value Projection: | USD 103.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Thermo Fisher Scientific Inc., Lonza Group, Merck KGaA, Corning Inc., Sartorius AG, Danaher Corporation, Miltenyi Biotec, Stemcell Technologies Inc., GE Healthcare, Takara Bio Inc., Bio-Techne Corporation, HiMedia Laboratories, Eppendorf AG, Avantor, Inc., Beckman Coulter, Inc., Terumo BCT, Inc., Roche Diagnostics, Irvine Scientific, Fujifilm Irvine Scientific, Inc., CellGenix GmbH |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
GMP Cell Therapy Consumables Industry News
- In January 2022, Sony Corporation launched the CGX10 Cell Isolation System, which sorts cells at high speed and purity within a closed, sterile environment. This system is crucial for cell-based immunotherapy, used in cancer and autoimmune disorder treatments, where high-purity, viable cells are needed.
- In January 2021, Sartorius entered into a strategic partnership with RoosterBio Inc., a prominent supplier of human mesenchymal stem/stromal cells (hMSC). This partnership focuses on scaling up hMSC manufacturing for regenerative medicine. RoosterBio and Sartorius developed a series of GMP-compatible, customer-focused protocols that combined RoosterBio’s hMSC and media systems with Sartorius’s single-use manufacturing technologies, process control software, and cell analysis tools for final hMSC product manufacturing.
- In September 2020, Bio-Techne Corporation inaugurated a state-of-the-art, approximately 61,000-square-foot Good Manufacturing Practices (GMP) facility in St. Paul, U.S. The facility is dedicated to large-scale production of GMP-grade materials, crucial for many immuno-oncology and regenerative medicine cell and gene therapy workflows. This facility enables Bio-Techne to meet the growing demand for GMP-grade reagents essential to the expanding cell therapy market.
*Definition: Global GMP cell therapy consumables market consists of consumable products and solutions used for cell therapy manufacturing under good manufacturing practices (GMP) guidelines. This includes cell culture media, reagents, containers, storage bags, tubes and other laboratory supplies that are all essential for the commercial scale manufacturing of cell therapy products in a regulated GMP environment to treat various medical conditions.
Market Segmentation
- Product Type Insights (Revenue, USD Mn, 2020 - 2032)
-
- Cell Culture Media and Supplements
- Cell Separation and Purification Consumables
- Cryopreservation Consumables
- Cell Transduction Consumables
- Other Consumables (Labware, Bioreactors, etc.)
- Cell Type Insights (Revenue, USD Mn, 2020 - 2032)
-
- Stem Cells (Embryonic, Induced Pluripotent, Adult)
- T-Cells
- Natural Killer (NK) Cells
- Other Cell Types (Dendritic Cells, Chondrocytes, etc.)
- Application Insights (Revenue, USD Mn, 2020 - 2032)
-
- Oncology
- Regenerative Medicine
- Neurology
- Cardiovascular
- Immunology
- Other Applications
- End User Insights (Revenue, USD Mn, 2020 - 2032)
-
- Biopharmaceutical Companies
- Contract Manufacturing Organizations (CMOs)
- Academic and Research Institutions
- Hospitals and Clinics
- Others
- By Regional Insights (Revenue, USD Mn 2020 - 2032)
-
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Thermo Fisher Scientific Inc.
- Lonza Group
- Merck KGaA
- Corning Inc.
- Sartorius AG
- Danaher Corporation
- Miltenyi Biotec
- Stemcell Technologies Inc.
- GE Healthcare
- Takara Bio Inc.
- Bio-Techne Corporation
- HiMedia Laboratories
- Eppendorf AG
- Avantor, Inc.
- Beckman Coulter, Inc.
- Terumo BCT, Inc.
- Roche Diagnostics
- Irvine Scientific
- Fujifilm Irvine Scientific, Inc.
- CellGenix GmbH
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
