The global troponin market was valued at US$ 1.38 Bn in 2025 and is expected to reach US$ 3.19 Bn by 2032, growing at a compound annual growth rate (CAGR) of 12.7% from 2025 to 2032.
Troponins are regulatory proteins that are integral to muscle contraction in skeletal and cardiac muscles. There are three troponin proteins - troponin T, troponin I, and troponin C. Troponin T binds tropomyosin to troponin C on the thin filament of muscle fibers. Troponin I inhibits the actomyosin ATPase activity of muscle fibers. Troponin C binds calcium and facilitates contraction by allowing interaction between actin and myosin.
Cardiac troponins specifically refer to as cTnT and cTnI are only expressed in the heart. When the heart is injured, these proteins leak into the bloodstream and can be detected usually within 3-4 hours of the start of symptoms. Measurement of cardiac troponin levels is now the recommended biochemical method for the diagnosis of myocardial infarction. cTnT and cTnI have nearly replaced creatine kinase-MB for this purpose due to their greater sensitivity and specificity for cardiac injury. They enable the detection of smaller infarcts and allow for early rule-out of myocardial infraction. However, elevated troponin levels can also occur in other cardiac conditions such as myocarditis, heart failure, pulmonary embolism, etc. so the clinical context is important in interpretation.
Global Troponin Market Regional Insights
- North America is expected to be the largest market for troponin over the forecast period, and it accounted for over 36.3% of the market share in 2025. The growth of the market in North America is attributed to the presence of leading medical device companies and an established healthcare infrastructure, thus driving the market growth over the years.
- Europe is expected to be the second-largest market for troponin, which accounted for over 30.4% of the market share in 2025. The growth of the market is attributed to the high healthcare spending.
- Asia Pacific is expected to be the fastest-growing market for troponin, which is expected to grow at a CAGR of over 8.2% during the forecast period. The growth of the market in Asia Pacific is attributed to the rapidly developing economies such as China and India, which are expected to showcase high investment opportunities owing to increasing healthcare expenditures and an expanding healthcare infrastructure.
Figure 1. Global Troponin Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample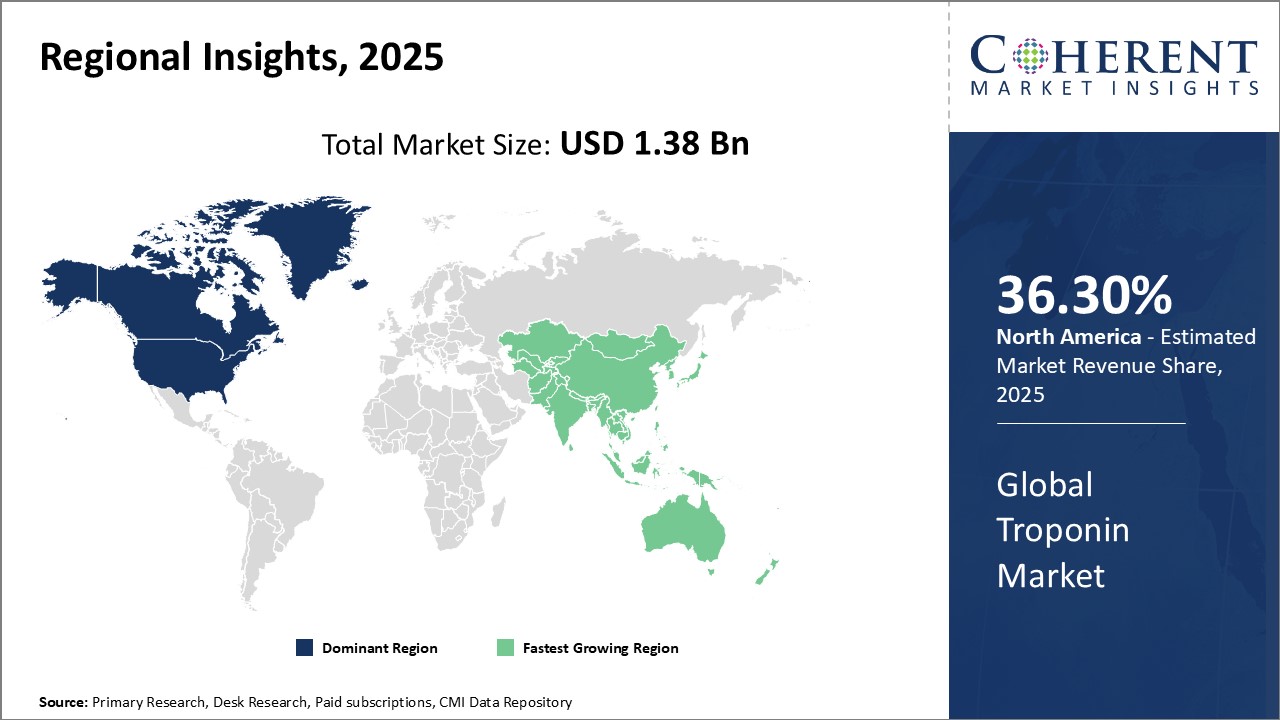
Analyst’s Views:
The global troponin market is expected to witness steady growth over the forecast period driven by the increasing incidence of cardiovascular diseases across major regions. Moreover, the rising geriatric population who are more prone to cardiac conditions will further propel the demand for troponin tests. However, stringent regulatory frameworks for the approval of troponin tests may hamper the market growth to some extent. North America will continue dominating the global troponin market owing to the well-established healthcare infrastructure and rising expenditure on cardiac biomarkers diagnostic tests in the region. Meanwhile, Asia Pacific excluding Japan is anticipated to depict the fastest growth and emerge as the most lucrative market by 2028. This can be attributed to growing healthcare awareness, increasing healthcare spending, and constant technological advancements in countries like China and India. The market in the region will continue receiving tailwinds from supportive government policies and initiatives towards quality healthcare.
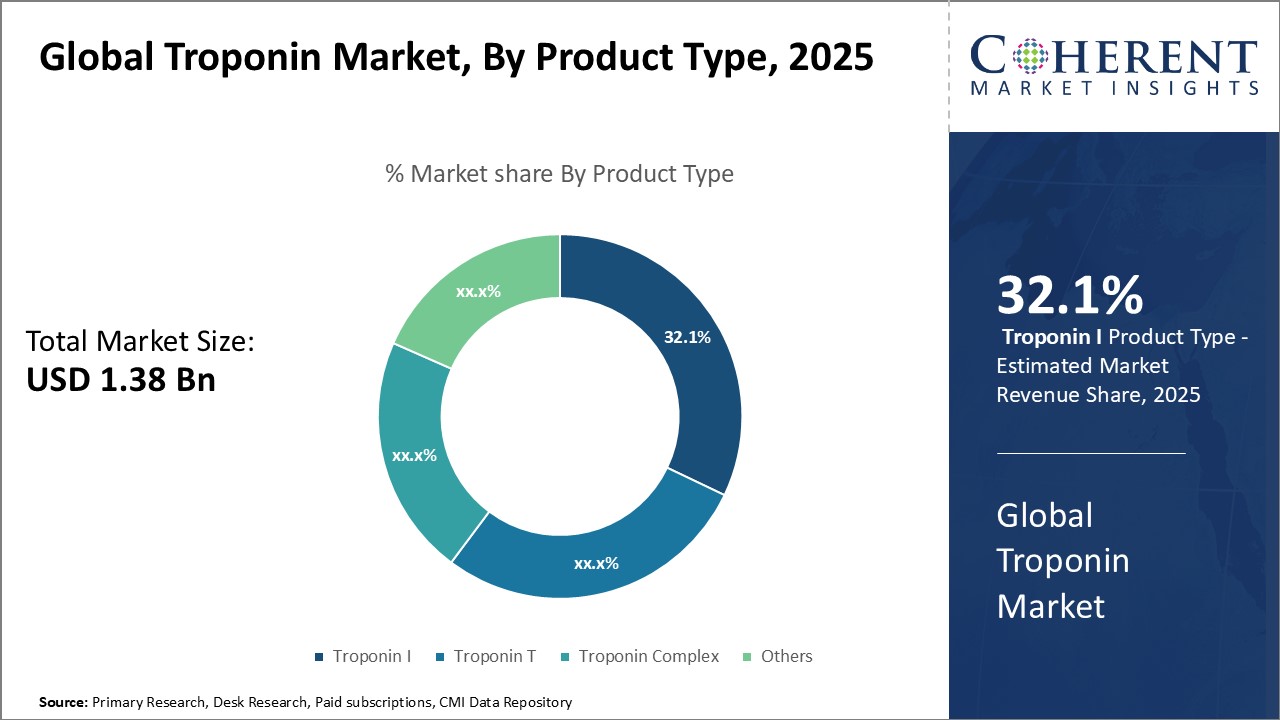
Among product type, troponin I and T together held over 60% share of the total market share in 2025. Cardiac intensive care units are expected to remain the key end users holding a revenue share of around 30% by the end of the forecast period. However, large hospitals and clinics will witness the fastest growth backed by the emergence of high throughput laboratories and the rising number of cardiovascular procedures in hospital settings.
Global Troponin Market Drivers:
- Increasing incidence of cardiac diseases: The increasing prevalence of cardiovascular diseases (CVDs) across the globe is one of the primary factors fueling the expansion of the global troponin market. According to an article published the World Health Organization (WHO) in 2020, cardiovascular diseases are the leading cause of deaths worldwide. According to the same source, in 2020 alone, over 17.9 million people succumbed to CVDs which accounted for an estimated 32% of deaths globally. The top causes of CVDs are heart attacks and strokes which often require urgent clinical diagnosis. Furthermore, the growing geriatric population prone to heart conditions also plays a significant role. According to United Nations data, the number of persons aged 65 years or over is projected to rise from 727 million in 2020 to over 1.5 billion in 2050. This trend will inevitably lead to a proportional increase in the incidence of conditions like stable angina, unstable angina, chronic heart failure, etc. which requires long term monitoring through sensitive troponin tests.
- Technological advancements in troponin testing: Technological advancements in cardiac troponin testing have significantly expanded the clinical utility of troponin assays, driving the growth of the global troponin market. Highly sensitive troponin tests have revolutionized the early detection of myocardial injury by accurately detecting troponin elevations within a few hours of the onset of symptoms. This has improved the management of acute coronary syndrome and facilitated rapid decision making regarding further treatment. For example, according to a 2020 publication by the National Institute for Health and Care Excellence (NICE), the use of high-sensitivity troponin tests reduced the time to rule out heart attack from 9 hours to 3 hours without compromising diagnostic accuracy.
- Increasing adoption of point-of-care testing: The growing preference for point-of-care cardiac testing is propelling the global troponin market forward. Conventional laboratory-based cardiac marker tests can take several hours to produce results. However, point-of-care tests provide rapid assessment of cardiac injury, which is invaluable for timely clinical decision making and adequate management of heart attacks. Troponin assays are being increasingly integrated into point-of-care testing devices and platforms that can be operated with ease in hospital emergency departments, physician offices, and even ambulances. This allows for quick rule-in or rule-out of myocardial infarction at the time and place of initial presentation.
- Several major hospital networks and healthcare systems around the world have already begun employing point-of-care troponin tests generated from blood samples as small as a finger-prick. According to Public Health England, increased utilization of finger-prick testing led to a 20% reduction in the median time to revascularization for heart attack patients in the country from 2010 to 2020. With results being available within 15-20 minutes, physicians can initiate effective treatments much faster compared to conventional methods. This has significantly improved patient outcomes and healthcare efficiency. Point-of-care testing also enables more widespread cardiac screening activities in community settings through mobile health units and paramedics, expanding access to diagnostics.
Global Troponin Market Opportunities:
- Development of automated lab systems for troponin testing: The development of automated lab systems for troponin testing could unlock significant opportunities in the global troponin market. Traditionally, troponin tests have been performed manually in laboratories, which can be time-consuming and labor-intensive. However, recent technological advancements have led to the development of fully-automated lab systems that can improve the efficiency and turnaround time for troponin testing. These automated systems utilize robotics and smart software to automate the entire workflow from sample insert to result reporting. They can analyze multiple patient samples simultaneously without human intervention. This helps eliminate manual errors and allows labs to scale up their testing capacities substantially. For example, some systems can process over 1,000 troponin tests in just 24 hours with minimal staff requirement. The increased throughput can help address growing diagnostic demands and reduce waiting periods for patients in emergency rooms.
- Launch of novel products with improved sensitivity and accuracy: The global troponin market has been growing steadily over the past few years owing to rising cardiac cases worldwide and increasing adoption of troponin tests in hospitals and diagnostic labs. However, players in this market have been focusing on developing novel products that can further enhance the diagnostic ability and accuracy of troponin tests. The launch of such innovative products has the potential to significantly boost the troponin market growth in the coming years. Products launched recently like the Siemens AG, a Germany-based multinational technology, Atellica IM immunoassay analyzer claims to detect troponin I and T at much lower concentrations compared to conventional assays. Preliminary studies show this improved analytical sensitivity allows for detection of troponin increases 6 hours earlier in patients experiencing a heart attack. Such advancements in diagnostic windows give medical professionals a head start in treatment, preventing damage to the heart.
Global Troponin Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.38 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.7% | 2032 Value Projection: | USD 3.19 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Roche Diagnostics, Abbott Laboratories, Beckman Coulter, Response Biomedical, Vitro, Siemens Healthcare GmbH, Radiometer Medical ApS, bioMérieux SA, Thermo Fisher Scientific, Mitsubishi Chemical Europe, and other prominent players |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Troponin Market Trends:
- Consolidation among key players: The global troponin market has been witnessing significant consolidation trends among key players in recent years. Major companies have been acquiring smaller competitors to expand their product portfolios and geographical reach. For instance, Roche Diagnostics, develops innovative products and services, acquired Spark Biomedicals, a leading U.S.-based wearable neurostimulation solutions, in 2022 to enhance its cardiac biomarker testing solutions. Similarly, Abbott Laboratories, an American multinational medical devices took over Abbott Rapid Diagnostics, a global manufacturer of rapid point-of-care diagnostic tests in 2021, which strengthened Abbott's diagnostics business. Such consolidation moves allow companies to combine complementary technologies and leverage synergies.
- Integration of AI and machine learning into troponin testing: The integration of AI and machine learning into troponin testing is having a profound impact on the global troponin market. Advancements in these technologies have enabled the development of high sensitivity troponin assays that can detect even minuscule elevation in troponin levels during the early stages of a suspected heart attack. These high sensitivity assays leverage machine learning algorithms during their design to identify unique biomarkers and develop tests that are able to distinguish normal ranges from negligible increases associated with acute coronary syndrome. For example, researchers from the National Institutes of Health (NIH), 2020, worked with several diagnostic companies to create an artificial intelligence model trained on troponin test results from over 30,000 patients. The model was able to identify subtle troponin patterns that indicated heart issues better than traditional methods.
- Shift towards the development of panel-based testing: The shift towards panel-based testing trend is having a significant impact on the global troponin market. Panel-based testing involves testing for multiple biomarkers simultaneously using a single patient sample, as opposed to performing individual tests sequentially. This provides several advantages over individual biomarker testing. This trend towards panel-based testing is driving considerable demand for troponin assays that can be integrated into biomarker panels. According to recent data published by the European Society of Cardiology, the share of troponin tests being performed as part of cardiac biomarker panels increased from 36% in 2018 to 46% in 2021 across European hospitals. Particularly, high sensitivity troponin assays that can reliably detect even small changes in troponin levels have become the assay of choice for most cardiac biomarker panels. Their inclusion enables more accurate rule-in and rule-out of acute myocardial infarction.
Global Troponin Market Restraints:
- Unfavorable reimbursement scenario: The unfavorable reimbursement scenario from public and private payers has been one of the major restraints for the growth of the global troponin market. Troponins are cardiovascular biomarkers used for diagnosis and risk-stratification of acute myocardial infarction and other conditions. However, the commercial prices of troponin assays are much higher than many other routine biochemical tests performed in hospitals and diagnostic laboratories. Public insurers in most countries have regulated reimbursement rates for cardiovascular diagnostic tests including troponin assays. The reimbursement rates often do not match up with the research and development costs incurred by the diagnostic kit manufacturers. This makes troponin testing less profitable for hospitals and laboratories. As a result, many healthcare facilities are hesitant to fully adopt high sensitivity troponin assays which can aid earlier diagnosis of heart attacks but are also more expensive. This prolongs the reliance on less accurate troponin I and T assays. Similarly, private insurance plans also control costs by imposing restrictions on coverage and frequency of troponin testing through step-edit criteria.
- High cost of advanced troponin tests: The high cost of advanced troponin tests is one of the major factors restraining the growth of the global troponin market. Troponin tests have become an important investigative tool for diagnosis and risk assessment of patients presenting with signs and symptoms of acute coronary syndrome. However, the advanced high sensitivity troponin assays that can accurately measure very low levels of troponins in blood are highly expensive for both patients and healthcare systems.
Recent Developments
Key Developments
- In October 2023, Mediport, a company that provides a service that monitors interstate transport companies, and GE HealthCare, an independent healthcare technology and diagnostics company, inaugurated the first of its kind One-Stop Clinic to serve cardiology patients. Patients visiting the clinic will receive a same-day diagnosis and care plan.
- In 2021, Roche Diagnostics, a company that develops innovative diagnostic products, launched a series of five new intended uses for two key cardiac biomarkers using the Elecsys technology: high sensitive cardiac troponin T (cTnT-hs) and N-terminal pro-brain natriuretic peptide test (NT-proBNP). These tests are used for accessing heart attacks in patients. Roche Diagnostics' introduction of five new intended uses for these existing, globally accepted diagnostic solutions means more people could benefit from improved cardiovascular diagnostics.
Figure 2. Global Troponin Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Top Companies in the Global Troponin Market
- Roche Diagnostics
- Abbott Laboratories
- Beckman Coulter
- Response Biomedical
- Vitro
- Siemens Healthcare GmbH
- Radiometer Medical ApS
- bioMérieux SA
- Thermo Fisher Scientific
- Mitsubishi Chemical Europe
- Other Prominent Players
Definition: A troponin test measures the level of troponin in a sample of blood. Troponin is a protein found in the cells of the heart muscle. Normally, troponin levels in blood are so low that only the most sensitive types of tests can measure them. But if the heart muscle is damaged, troponin leaks into the bloodstream, increasing the troponin blood levels. It is used to confirm if a person is having a heart attack or recently had a heart attack, diagnose and monitor unstable angina and check heart health after a surgery that could damage the heart.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




