Global Synthetic Biosensors Market Size and Trends
The synthetic biosensors market is estimated to be valued at USD 30.04 Bn in 2025 and is expected to reach USD 49.87 Bn by 2032, growing at a compound annual growth rate (CAGR) of 7.5% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The synthetic biosensors market is witnessing high growth due to the rising demand for point-of-care testing and home healthcare devices. Synthetic biosensors find widespread application in industries like healthcare, food technology, water monitoring, and environmental protection due to their high selectivity and sensitivity. Advancements in nanotechnology and development of wearable and flexible biosensors are expected to drive the demand for synthetic biosensors. Growing healthcare expenditure along with increasing focus on drug delivery and disease diagnostics will further augment the market growth during the forecast period. High investment in the research & development of biosensors for new application areas ensures a bright growth opportunity for synthetic biosensors going ahead.
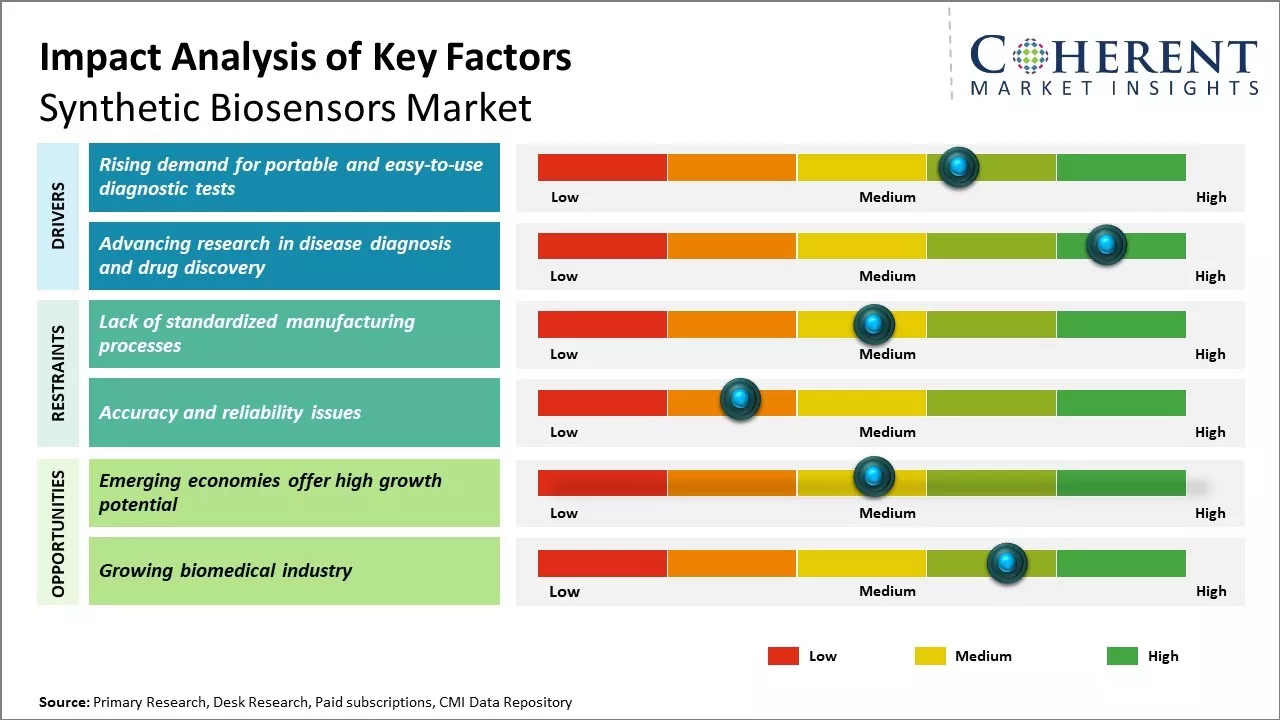
Rising demand for portable and easy-to-use diagnostic tests
The demand for point-of-care testing and home diagnostics is growing rapidly mainly due to hectic lifestyles and increased health awareness among consumers. People are increasingly looking for portable diagnostic devices that can detect a medical condition quickly and easily, without needing to visit a laboratory. Synthetic biosensors have the potential to enable portable diagnostic devices as they are small, affordable, and do not require sophisticated equipment to operate. The introduction of wearable biosensors and the integration of biosensors in smartphones present an opportunity to develop easy-to-use and non-invasive testing options that can be used anywhere, even in home settings. This has increased the appeal of synthetic biosensors which are being engineered to function accurately in portable, low-cost devices used outside of hospitals and diagnostic centers. With further technological advancements, synthetic biosensors are expected to open up new avenues for convenient, user-friendly healthcare monitoring and testing.
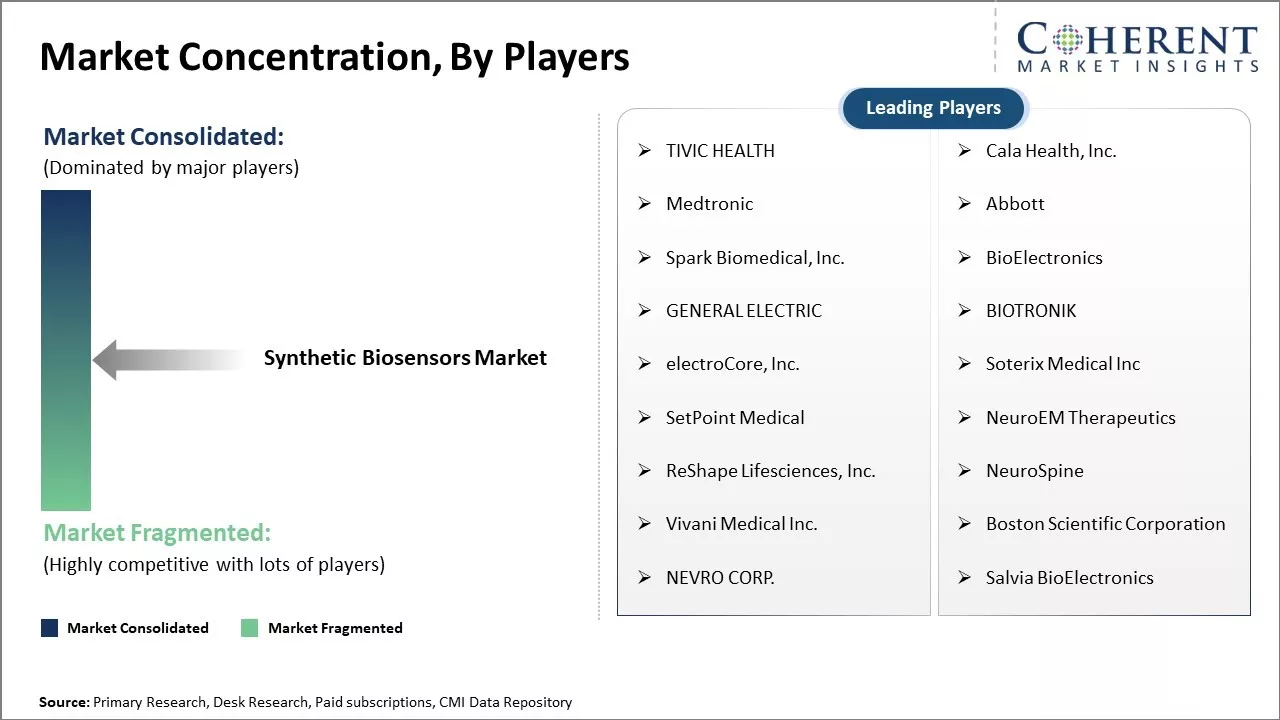
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Advancing research in disease diagnosis and drug discoverySynthetic biology is advancing research in areas like disease diagnosis and treatment, leading to higher investments in synthetic biosensor development. The ability of biosensors to rapidly detect multiple biomarkers associated with diseases at the molecular level allows for more precise diagnostics compared to conventional methods. This encourages researchers to design novel biosensors that can detect disease signatures with high accuracy. Similarly, biosensors play a crucial role in accelerating drug discovery by screening large libraries of chemical compounds for potential drug candidates. They enable evaluating interactions between target molecules and drug candidates in real-time, reducing dependency on time-consuming animal testing. The value of biosensors in research applications is a driving influential force as continued investments flow towards developing assay platforms and detection technologies centered around synthetic biology.

To learn more about this report, Download Free Sample
Market Challenges: Lack of standardized manufacturing processesOne of the major challenges the synthetic biosensors market faces is the lack of standardized manufacturing processes. Each biosensor needs to be tailor-made for specific sensing applications which makes mass production difficult. Another challenge is the limited lifetime and shelf life of these biosensors. Many synthetic biosensors degrade relatively quickly which hinders long-term usability. Maintaining high accuracy and repeatability of measurements also poses a challenge due to variability in biological and chemical reactions involved. Regulatory hurdles for approval of new biosensor technologies introduce time delays in commercialization.
Market Opportunities: Emerging economies offer high growth potential
Emerging economies are showing a lot of promise for growth in the synthetic biosensors market. As countries like India, China, Brazil, and others continue to develop economically, their populations are gaining more access to healthcare and biological monitoring tools. This rising access to such tools in middle-income nations is driving significant interest in low-cost, user-friendly synthetic biosensors. Synthetic biosensors offer a convenient alternative to traditional diagnostic tests in these developing markets. They provide portable, easy-to-use detection of various biomarkers and biomolecules. This makes them well suited for point-of-care testing and screening programs in remote or resource-limited areas that often lack advanced laboratory infrastructure.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
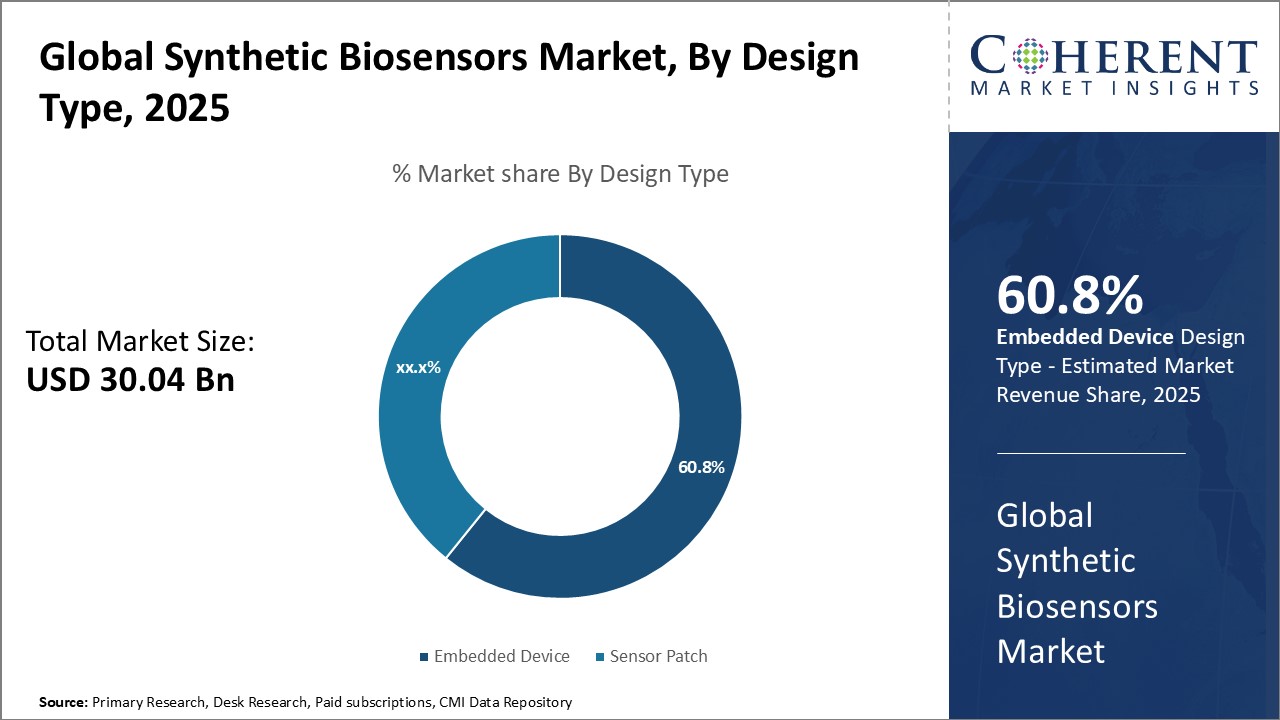
Insights, By Type: Embeddable Design and Functions Drive High Market Share of Embedded DevicesThe type segment includes healthcare sensor patch and embedded device. The embedded device segment is anticipated to hold 60.8% of the market share in 2025. Being integrated circuits or microcontrollers, embedded devices can be easily placed inside the host device or equipment whose parameters are required to be monitored. This embedded design allows for continuous, real-time sensing without any protruding part from the host. Many industries prefer embedded biosensors as they do not obstruct the regular functions of the machine and can seamlessly blend in. Embedded devices are also capable of measuring multiple parameters simultaneously through their array of sensors. This aids in comprehensive monitoring and analysis and makes them favorable for applications requiring concurrent testing of different biomarkers. Further, embedded biosensors have reprogrammable capabilities through which their sensing functions can be adapted as per changing requirements. This feature sustains their long-term usability.
Insights, By Modality: Convenience drives higher Adoption of Wearable Modality
The modality segment includes non-wearable and wearable. Wearable contributed the highest share of the synthetic biosensors market and is projected to hold 58.3% of the market share in 2025. Wearable biosensors are ergonomically designed accessories that can be effortlessly worn on the body like watches, patches or implants. This allows continuous health tracking even while being on the move. As lifestyle diseases are rising and preventive healthcare gaining prominence, wearable modality is becoming highly desirable. It empowers individuals to closely self-monitor vital signs and symptoms simultaneously pursuing daily activities. Further, data from wearables can be seamlessly synced with smartphones or linked online doctor consultations for remote monitoring. Their non-invasive nature safeguards repeated usage without any pain or hassle.
Insights, By Technology: High Sensitivity and Selectivity Drives the Demand for Electrochemical Technology
The technology segment includes piezoelectric, optical, electrochemical, and others. The electrochemical segment contributed the highest share of the synthetic biosensors market and is projected to hold 39.3% of the market share in 2025. Electrochemical biosensors detect and measure biomarkers by converting a biological response triggered by the analyte into an electrical signal. This is done using the redox potential created due to oxidation-reduction reactions between the analyte and an electrocatalyst. Being an amplification technique, electrochemical responses are greatly magnified resulting in excellent sensitivity to detect trace amounts of molecules. Moreover, different analytes can be monitored selectively by tuning the catalyst makeup. This targeted detection prevents signal interference from other compounds. Electrochemical biosensors are also eco-friendly as they do not involve complex optical or thermal components. Leveraging their advantages, electrochemical technology is highly sought-after in industries like healthcare, food testing, and environmental monitoring.
Regional Insights
Need a Different Region or Segment? Download Free Sample
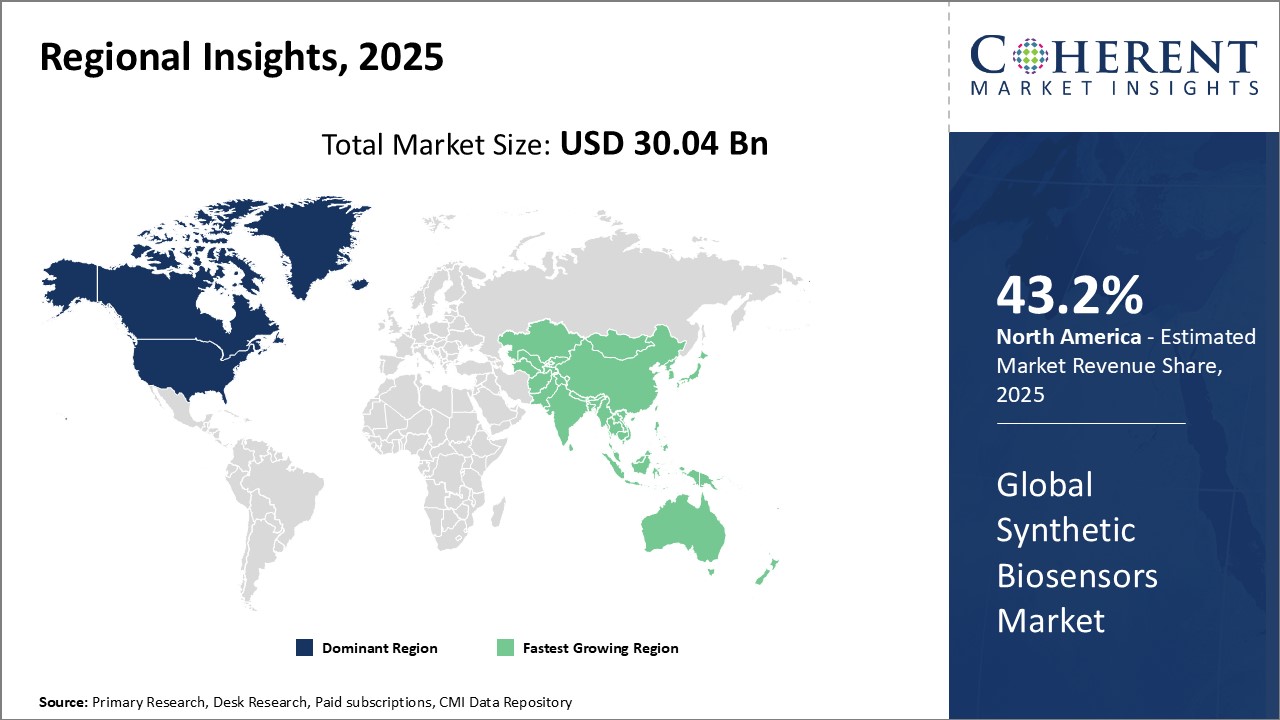
North America has firmly established itself as the dominant region in the global synthetic biosensors market and is projected to hold 43.2% of the market share in 2025. This can largely be attributed to the strong presence of leading players and their extensive R&D investments in the region. Several major companies manufacturing and developing biosensors, such as Abbott, Thermo Fisher Scientific, and Danaher, are headquartered in the U.S., giving North America an edge in terms of technology and innovation. The region also has a highly developed healthcare infrastructure and supportive regulatory environment conducive for the growth of the biosensors industry.
The Asia Pacific region, on the other hand, is emerging as the fastest growing market for synthetic biosensors globally. This can be accredited to rising healthcare expenditures, growing aging population demanding better healthcare services and increasing investments by both private and public players to strengthen the regional healthcare infrastructure and research. Countries like China, Japan, and South Korea have large and growing medical devices industries actively investing in developing localized synthetic biosensor technologies. For instance, China is providing significant funding through initiatives like the National Key R&D Program to promote indigenous research on biosensors. Additionally, the low manufacturing costs and readily available technical expertise in the region is attracting several global leaders to establish manufacturing bases in Asia Pacific to cater to the worldwide demand.
Market Report Scope
Global Synthetic Biosensors Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 30.04 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.5% | 2032 Value Projection: | USD 49.87 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
TIVIC HEALTH, Cala Health, Inc., Medtronic, Abbott, Spark Biomedical, Inc., BioElectronics, GENERAL ELECTRIC, BIOTRONIK, electroCore, Inc., Soterix Medical Inc, SetPoint Medical, NeuroEM Therapeutics, ReShape Lifesciences, Inc., NeuroSpine, Vivani Medical Inc., Boston Scientific Corporation, NEVRO CORP., and Salvia BioElectronics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Synthetic Biosensors Industry News
- On January 31, 2024, the World Health Organization (WHO) and the Medicines Patent Pool (MPP), a United Nations-backed public health organization, announced a license agreement with SD Biosensor Inc., a global in-vitro diagnostic company, to grant sublicensee the right, know-how, and materials to manufacture SDB's rapid diagnostic testing (RDT) technology
- In August 2023, IdentifySensors Biologics, a leading developer of digital diagnostic technologies, partnered with U.S.-based, East West Manufacturing, a global engineering and manufacturing services company, to create medical diagnostic devices capable of rapidly detecting and distinguishing multiple infections from a single saliva sample. The testing platform employs printed solid-state electronic biosensors designed to quickly detect pathogen DNA and RNA in saliva samples.
- In April 2022, FREDsense Technologies Corp, a next-generation water quality platform firm, and Ginkgo Bioworks, the leading horizontal platform for cell programming, announced a partnership to develop biosensors for water quality monitoring and detection. Through this partnership, Ginkgo Bioworks seeks to create four separate microbial strain biosensors that are compatible with FREDsense Technologies Corp's field-ready hardware for remote water quality monitoring applications.
- In May 2020, Royal Philips, a global leader in health technology, announced that it has acquired 510(k) approval from the U.S. Food and Drug Administration (FDA) for its wearable biosensor (Philips Biosensor BX100) to help manage proven and suspected COVID-19 patients in the hospital
*Definition: The synthetic biosensors market involves sensors that incorporate biomolecular recognition elements with synthetic materials and nanotechnology to detect biological and chemical stimuli. These biosensors transform biological response into quantifiable signals for detecting a wide range of compounds including heavy metals, drugs, proteins, DNA, viruses, and bacteria.
Market Segmentation
- Design Type Insights (Revenue, USD BN, 2020 - 2032)
- Sensor Patch
- Embedded Device
- Modality Insights (Revenue, USD BN, 2020 - 2032)
- Non-wearable
- Wearable
- Technology Insights (Revenue, USD BN, 2020 - 2032)
- Piezoelectric
- Optical
- Electrochemical
- Others
- Application Insights (Revenue, USD BN, 2020 - 2032)
- Home Diagnostics
- Research Labs
- Point-of-care Testing
- Others
- Regional Insights (Revenue, USD BN, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- TIVIC HEALTH
- Cala Health, Inc.
- Medtronic
- Abbott
- Spark Biomedical, Inc.
- BioElectronics
- GENERAL ELECTRIC
- BIOTRONIK
- electroCore, Inc.
- Soterix Medical Inc
- SetPoint Medical
- NeuroEM Therapeutics
- ReShape Lifesciences, Inc.
- NeuroSpine
- Vivani Medical Inc.
- Boston Scientific Corporation
- NEVRO CORP.
- Salvia BioElectronics
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
