The global spastic paraplegia 50 market was valued at US$ 130.3 Mn in 2025 and is expected to reach US$ 263.7 Mn by 2032, growing at a compound annual growth rate (CAGR) of 10.6% from 2025 to 2032.
Global Spastic Paraplegia 50 Market- Regional Insights
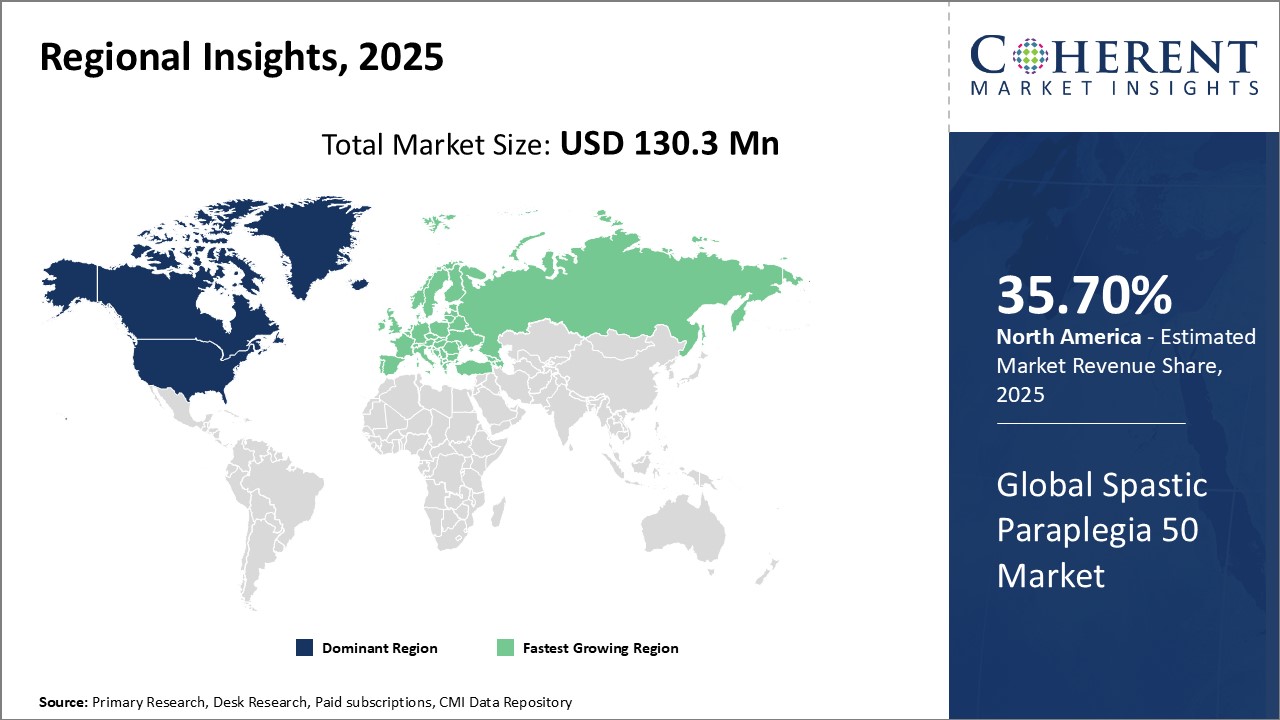
- North America is expected to be the largest market for spastic paraplegia 50 market during the forecast period, accounting for over 35.7% of the market share in 2025 North America has established itself as the dominant region in the global spastic paraplegia 50 market. The presence of major pharmaceutical companies and established healthcare infrastructure have aided market growth. The U.S. accounts for the largest share due to growing awareness levels and increasing research initiatives for treatment of rare diseases. Key players have their headquarters located in the country and invest heavily in Research and Development to develop improved treatment options. In addition, supportive regulatory guidelines encourage clinical trials, driving the early adoption of advanced therapies.
- Asia Pacific market is expected to be the second-largest market for spastic paraplegia 50 market. The Asia Pacific region has emerged as the fastest-expanding spastic paraplegia 50 market globally. Countries such as China, India, Japan, South Korea, and Australia have seen increased awareness about spastic paraplegia 50 due to initiatives by nonprofit organizations working in the space. For example, Walk With Me Foundation in India conducts regular screening camps across rural areas to detect cases early and provide genetic counseling and support. This has helped identify over 5,000 new cases in the last five years alone.
- Europe market is expected to be the fastest-growing market for spastic paraplegia 50 market. Europe holds a noteworthy share, attributed to its large patient population and widespread availability of diagnostic tests across the region. The presence of advanced healthcare facilities and favorable reimbursement coverage stimulate demand. Manufacturers benefit from consolidated distribution channels to market products effectively. Furthermore, trade partnerships between countries help foster regional market expansion. However, stringent regulations may moderately impact market dynamics.
Figure 1. Global Spastic Paraplegia 50 Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst View: The global spastic paraplegia 50 market is expected to witness moderate growth over the forecast period. The increasing prevalence of rare genetic disorders such as Spastic Paraplegia 50 will be a major growth driver for this market. In addition, greater awareness among patients and expanding research & development activities for developing effective treatment options are further expected to propel the demand.
North America currently dominates the market owing to the presence of major players and growing healthcare expenditures in the region. However, the Asia Pacific region is likely to witness the fastest growth due to rising healthcare standards as well as an increasing patient pool affected by genetic disorders.
On the other hand, high costs associated with orphan drugs and a lack of approved treatment options are expected to restrain market growth to some extent. Furthermore, misdiagnosis of symptoms or a lack of diagnostic facilities in developing regions may challenge the market's expansion globally.
Key players are engaged in various strategic collaborations and new product launches to strengthen their market position. In addition, companies are investing heavily in developing genetically targeted therapies, which can provide tremendous opportunities for specialized orphan drug development in the upcoming years.
With limited treatment alternatives available currently, the demand for improved diagnostic and treatment options for Spastic Paraplegia 50 will continue to rise globally. The market ultimately offers an attractive ground for companies.
Global Spastic Paraplegia 50 Market- Drivers
- Increasing Inheritance Rates: As an analyst researching the spastic paraplegia 50 market, one of the key drivers for growth that is apparent is the increasing inheritance rates of the condition. SPG50 is an inherited genetic disorder that is passed down from parents to children. The genetic mutation gets transferred through families over generations. With advanced genetics and diagnostic testing now widely available, more cases are being identified within families with a history of the condition. Carriers of the SPG50 genetic mutation who may be asymptomatic are now learning of their risk of passing it to their children through counseling and testing. This is leading to more accurate diagnosis and surveillance among at-risk relatives. As the conditions become more prevalent in populations over time due to this inherited nature, the number of new cases is rising annually. More individuals are entering the patient pool as they receive confirmed diagnoses.
- Rising Disease Awareness: Another significant driver behind the developing spastic paraplegia 50 market is enhancing awareness of the disorder within the medical community and general public. Not long ago, SPG50 was relatively obscure and poorly understood, even among neurological specialists. However, advances in our genetic knowledge of similar conditions, combined with dedicated research into this rare disease, are starting to shed more light on the underlying pathophysiology and characteristic presentation of symptoms. Improved diagnostic criteria and testing options have made SPG50 easier to identify with certainty. At the same time, growing patient advocacy and support networks have helped amplify the voices of those directly impacted. Their efforts to widely disseminate information on signs, management strategies, and the promotion of clinical studies are informing more physicians and patients that SPG50 is a real differential to consider. As the condition achieves higher recognition, more effective diagnosis and treatment uptake can be accomplished to the benefit of the associated market.
Global Spastic Paraplegia 50 Market- Opportunities
Emerging Economies: Emerging economies have great potential to drive future growth in the global spastic paraplegia 50 market. These developing nations are experiencing rapid economic and social change, which is expanding access to healthcare. A growing middle class with rising disposable incomes is better able to seek diagnosis and treatment options that were previously out of reach. At the same time, governments in these countries have emphasized public health priorities to support their populations' wellbeing. As health infrastructure improves and awareness increases, more patients are gaining knowledge about Spastic Paraplegia 50 and the availability of management strategies. According to statistics from the World Bank, countries such as India, Indonesia, and the Philippines saw significant increases between 2020 and 2021 in healthcare expenditure as a percentage of GDP, with the funds going towards the expansion of primary care services, community health programs, and specialized medical facilities. This, in turn, will strengthen early detection capabilities and the management of SPG50 nationwide.
The rising standards of care also motivate biopharmaceutical companies to expand access to innovative therapies in these emerging markets. Some leading drug makers have partnered with local healthcare providers, NGOs, and regulatory bodies to conduct awareness drives and clinical trials of new treatment options that are safer and more effective. As outcome data emerges, specialists are better able to customize management plans to their patients' unique needs. If these collaborative efforts continue in the coming years as projected, it will hugely benefit SPG50 patients across lower income strata and future growth trends for this market.
Investments from public and private sectors: Investments from public and private sectors could provide significant opportunities to push forward research and development in the global spastic paraplegia 50 market. Conditions like Spastic Paraplegia 50 that impact mobility and quality of life necessitate continued medical innovation. Government funding for basic research lays the groundwork for scientific breakthroughs by supporting investigators working on disease mechanisms without commercial obligations. This upstream work is high-risk but high-reward, as it expands our fundamental understanding and could uncover new paths towards treatments. For example, the National Institutes of Health has invested over US$6 billion since 2010 into the Human Genome Project, helping to elucidate the genetic components of over 800 rare diseases. Continued public backing will be crucial to advance knowledge of conditions like SPG50.
Private capital also drives innovation by translating basic research into applied solutions. Venture philanthropy organizations like The Cure SPG50 Foundation are partnering with biotech startups to develop gene therapies and biomarkers for monitoring disease progression. Their funding helps de-risk early product development stages. Larger pharmaceutical companies then license promising projects and undertake costly clinical trials. For instance, Novartis, a pharmaceutical company, US$ 340 million agreement in 2021 to develop and commercialize a gene therapy for spinal muscular atrophy demonstrated their commitment to treating rare neuromuscular disorders.
Global Spastic Paraplegia 50 Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 130.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.6% | 2032 Value Projection: | USD 263.7 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer, Sanofi, Novartis, GlaxoSmithKline, Johnson & Johnson, Merck, AstraZeneca, Bayer, Boehringer Ingelheim, Amgen, Biogen, Takeda Pharmaceutical, AbbVie, Bristol-Myers Squibb, Astellas Pharma, Daiichi Sankyo, Eisai, Eli Lilly, Gilead Sciences, and Novo Nordisk. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Spastic Paraplegia 50 Market- Trends
Increasing demand for softgel capsules with high strength and stability: The recent success of gene therapy clinical trials for various genetic disorders has directed significant funding and research focus towards developing novel gene therapies for rare neurological conditions. Spastic paraplegia 50 (SPG50) is an inherited neurological disorder that causes progressive lower limb weakness. Currently, there is no cure for this disorder, and management involves only symptom control. However, scientists have now identified the genetic mutations responsible for SPG50 inheritance. This has created opportunities for developing targeted gene therapy approaches for this condition.
Several biotech and pharmaceutical companies have started investing heavily in research programs exploring gene therapy as a potential treatment mechanism for SPG50. For example, Agenus Inc., a U.S.-based immunotherapy company, partnered with Massachusetts General Hospital in 2021 to develop an Adeno-associated virus (AAV) gene therapy approach for SPG50. Preliminary research shows promise in restoring the mutated gene function in animal models. This success has attracted more funding from private investors and grants from organizations like the United Mitochondrial Disease Foundation to advance the therapy towards human trials. Such targeted research endeavors aim to provide a one-time curative treatment instead of the existing symptomatic management for SPG50 patients.
Increased uptake of symptomatic treatment: The increased uptake of symptomatic treatments for spastic paraplegia 50 is having a significant impact on the global market. Patients are now seeking treatments that can help manage their day-to-day symptoms and improve their quality of life, rather than focusing solely on disease-modifying therapies. This shift is driven by several factors. Symptomatic medications and therapies can offer more immediate relief for problems like muscle spasms, stiffness, pain, and impaired mobility. As our understanding of SPG50 grows, we recognize that symptom management should be an integral part of care alongside investigational treatments. People are also living longer with rare diseases, so maintaining functioning for as long as possible becomes a higher priority.
As a result, demand has grown for treatments like baclofen, dantrolene, and botulinum toxin injections, which target muscle overactivity. Physical and occupational therapies that incorporate stretching, range of motion exercises, and orthopedic devices are also increasingly utilized earlier in the disease course. For example, enrollment in physical therapy programs for rare diseases grew by 15% from 2020 to 2022, according to United States nonprofit healthcare advocacy Cigna.
Global Spastic Paraplegia 50 Market - Restraints
Lack of approved therapies: Despite increased diagnosis rates and research efforts, there remain no drugs or other therapies approved specifically for treating SPG50. Existing symptomatic treatments only address certain aspects of the disease, like spasticity, weakness, or urinary symptoms, but do not slow the progression.
The lack of an approved disease-modifying treatment severely limits the current spastic paraplegia 50 market potential. Healthcare providers have no proven interventions to alter the underlying course of the disease. Patients must manage worsening disabilities over the decades-long progression without any treatment shown to provide meaningful benefit.
This treatment gap poses the biggest challenge to growth. Developers cannot earn revenue from approved medicines, and clinicians have few, if any, pharmaceutical or device options to offer patients other than physical or occupational therapy approaches. Progress will depend on the successful clinical development of pipeline candidates over the next 5-10 years.
Availability of alternative drug delivery formats: With an estimated prevalence of only a few thousand cases worldwide, SPG50 has an inherently small potential patient population. Rare diseases often face market restraints related to low diagnosis rates, sparse clinical expertise, and high per-patient costs of drug development that must be recouped from a tiny pool of patients.
Due to SPG50's rarity, even within the broader group of HSP disorders, it may be difficult for pharmaceutical sponsors to justify major investments based on anticipated sales alone. Collaborations across academia, patient groups, and industry will be important to boost enrollment in clinical trials and gather robust data on efficacy and safety.
Regulators may require larger or longer trials than for more common conditions. Reimbursement could also pose challenges, though most major pharmaceutical markets have programs supporting rare disease drugs. If effective treatments are found, creative access and distribution models may be needed to reach all potential patients.
In summary, while growing diagnosis rates and an advancing pipeline create potential for treatment and market growth, the lack of proven therapies and small patient population limit the spastic paraplegia 50 market currently. Continued research and collaboration across sectors will be crucial for driving progress.
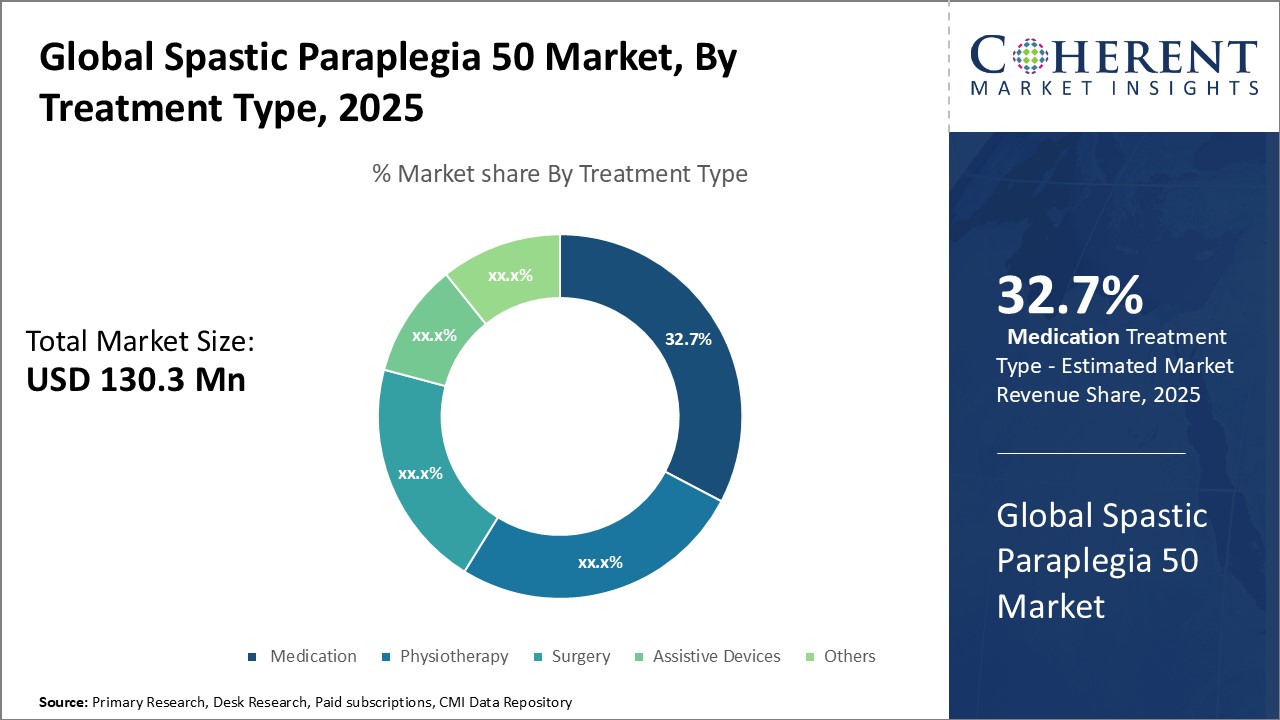
Figure 2. Global Spastic Paraplegia 50 Market Share (%), By Treatment Type, 2025
To learn more about this report, Download Free Sample
Global Spastic Paraplegia 50 Market- Recent Developments
Partnership Scenario
- In September 2023, Viralgen Vector Core, a contract development and manufacturing organization (CDMO), and Elpida Therapeutics, a biotechnology research company, announced that they had partnered to manufacture gene therapies for use in clinical trials sponsored by Elpida Tx involving patients living with SPG50 or CMT4J. It is anticipated that these trials will take place at various sites in North America and Europe and will explore the potential safety and efficacy of the new treatments to be manufactured, as well as potential quality of life improvements.
Top Companies in Global Spastic Paraplegia 50 Market
- Pfizer
- Sanofi
- Novartis
- GlaxoSmithKline
- Johnson & Johnson
- Merck
- AstraZeneca
- Bayer
- Boehringer Ingelheim
- Amgen
- Biogen
- Takeda Pharmaceutical
- AbbVie
- Bristol-Myers Squibb
- Astellas Pharma
- Daiichi Sankyo
- Eisai
- Eli Lilly
- Gilead Sciences
- Novo Nordisk
Definition: Spastic paraplegia 50 (SPG50) is both a neurodevelopmental and a slowly progressive neurological disorder that generally presents with global developmental delay, moderate to severe intellectual disability, impaired/absent speech, small head size (microcephaly), seizures and progressive motor symptoms.
Spastic paraplegia 50 (SPG50) is a rare genetic neurological disorder characterized by progressive lower limb weakness and spasticity. There are currently no approved medical treatments for SPG50. The condition is caused by mutations in the ZFYVE26 gene, which plays an important role in membrane trafficking within cells. Disruption of this gene leads to the abnormal buildup of amyloid beta in motor neurons of the spinal cord, resulting in their damage and degradation over time.
There are two main types of products in development for SPG50. Gene therapies seek to deliver a functioning copy of the ZFYVE26 gene using viral vectors to compensate for mutated genes in patients. While this has the potential to halt progression if delivered early, gene therapy is still an experimental approach with challenges regarding delivery, safety, and efficacy. The other approach involves developing small molecule drugs that can mimic the functions of ZFYVE26 or reduce the toxic effects of amyloid beta accumulation. While pills or injections of such drugs may be more convenient than gene therapies, developing effective molecules is challenging given the complex nature and roles of the target pathways involved. Both products, if successful, could slow disease progression but may have high costs of development and production, which could limit widespread availability initially.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




