Global Small Volume Parenteral Market is estimated to be valued at USD 207.57 Bn in 2025 and is expected to reach USD 344.37 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.5% from 2025 to 2032.
Small volume parenteral (SVPs) are prefilled syringes or vials containing sterile solutions for injection or infusion. SVPs are typically defined as containing volumes less than or equal to 100 milliliters and are primarily used for drug reconstitution or direct intravenous administration. There are two main types of SVPs - syringes and vials.
SVPs offered as prefilled syringes have several advantages over conventional vials. Syringes provide a single-dose format that eliminates the step of withdrawing the drug from a vial, thus reducing the risk of contamination as compared to using vials. They also allow for accurate dosing as the injection volume is fixed. This can be important for drugs with a narrow therapeutic index. From a production standpoint, syringes are easier to manufacture aseptically than vials. However, syringes are generally more expensive than vials. Vials remain a popular format as they are convenient for withdrawing multiple doses. But vials require reconstitution of the dry powder, which can be time-consuming and introduces opportunities for errors.
Global Small Volume Parenteral Market- Regional Insights
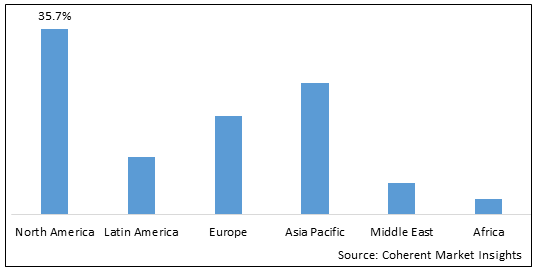
- North America is expected to be the largest market for small volume parenteral during the forecast period, accounting for over 35.7% of the market share in 2025. North America has established itself as the dominant region in the global small volume parenteral market. With a strong presence of leading pharmaceutical companies and contract manufacturing organizations, the U.S. and Canada account for over 40% of the worldwide market. The regional market benefits from well-established healthcare and distribution infrastructure, which ensures reliable supplies to meet medical needs. Favorable regulations promote innovations as North American firms can quickly commercialize new formulations. This first-mover advantage allows them to export products globally and maintain leadership.
Another major factor for North America's prominence is pricing flexibility. Healthcare providers regularly evaluate products and easily switch between offerings from regional or international firms. This competitive environment encourages continuous upgrades and cost optimizations by manufacturers. With the ability to quickly adapt to changes, North American companies protect their dominance by providing options that offer the best value propositions.
- Asia Pacific market is expected to be the second-largest market for small volume parenteral market, accounting for over 25.2% of the market share in 2025. The Asia Pacific region has emerged as the fastest growing market for small volume parenteral. Rapid economic development and improving accessibility to healthcare across nations like China, India, and Southeast Asia are driving heavy demand increases. The vast patient pools and growing medical expenditures provide significant opportunities. While regulations and capabilities vary significantly across countries, Asia Pacific nonetheless witnesses strong volume growth. India in particular has become an attractive manufacturing hub, with low-cost skilled labor and a large pipeline of generics. Indian firms leverage their formulation expertise and low-cost structures to efficiently cater to both domestic needs as well as export opportunities across Asia, Africa, and South America. Export volumes are rising sharply as international markets recognize the quality and reliability of Indian manufactures. This is propelling the overall regional market expansion.
- Europe market is expected to be the fastest-growing market for small volume parenteral market, with a market share of 19% during the forecast period. The growth of the market in Europe is due to the increasing prevalence of diabetes in the region.
Analyst View: Global small volume parenteral market is expected to grow significantly in the near future. The market is driven by rising prevalence of chronic diseases and increasing demand for convenient drug delivery formulations. Growing geriatric population susceptible to various chronic conditions is further expected to propel the demand. Advancements in parenteral drug development with minimum drug waste and improved stability of ingredients at small volumes will aid the market growth. However, stringent regulations pertaining to sterile prefilled syringes and high costs associated with parenteral drug development pose challenges to market players. Safety concerns regarding injection devices could also restrain the market growth. Furthermore, limitations in drug ingredients that can be formulated for small volumes restrict the applications.
Figure 1. Global Small Volume Parenteral Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Small Volume Parenteral Market- Drivers
- Increasing geriatric population: Global population is aging at an unprecedented rate. For instance, on May 29, 2023, according to a report published by the National Center for Biotechnology Information, in 2022, there were approximately 771 Mon people aged 65+ years globally, accounting for almost 10% of the world’s population, and this figure has been growing at an increasing rate, which is expected to hit 16% in 2050, and eventually 24% by 2100. An aging population drives demand for healthcare services across the board. The population over 60 years of age is more susceptible to chronic medical conditions such as cardiovascular diseases, diabetes, cancer, and respiratory illnesses which require long term treatment. This long term treatment involves frequent doses of medications administered through small volume parenteral routes such as injections to ensure strict adherence to treatment regimes. Anti-aging therapies involving nutrition supplements and cosmetic procedures also contribute to the growing demand for parenteral medications in the geriatric segment.
- Shortage of drug therapies: There is a persistent shortage of available drug therapies and treatment options for certain therapeutic areas. Particularly areas like oncology, rheumatology and chronic inflammatory diseases have limited pipeline of new drug innovations. At the same time, requirements for specialized treatment have been on the rise. This supply-demand imbalance has compelled healthcare practitioners to seek alternative treatment delivery methods. Small volume parenteral administration of existing off-patent drugs has emerged as a viable solution to fulfill unmet medical needs. However, manufacturing and distribution of such therapies involves higher costs and strict production standards. Still, it allows for re-purposing of drugs, bridging treatment gaps, and consequently expanding the market for small volume parenteral pharmaceuticals. This force of necessity will continue driving adoption of small volume parenteral drugs by healthcare professionals.
- Increasing uptake of biologics: Increasing uptake of biologics in the global small volume parenteral market is a response to the growing emphasis on precision medicine and targeted therapies, which biologics are well-suited to address. Small volume parenteral formulations are typically defined as those presented in volumes of 100 mL or less and can include several types of drugs, including biologics. Here are the reasons and implications for the rise in biologics within the SVP market:
- Enhanced Efficacy and Specificity:
- Targeted Therapies: Biologics are often designed to target specific cellular receptors or pathways, thus making them highly effective for certain diseases.
- Reduced Side Effects: Due to their specificity, biologics can have fewer side effects as compared to some broad-spectrum pharmaceuticals
- Growth in Chronic Diseases:
- Demand for Treatment: There is an increased prevalence of chronic diseases such as diabetes, rheumatoid arthritis, and multiple sclerosis, many of which are better managed by biologic drugs.
- Specialized Medications: Biologics provide options for patients with conditions that do not respond well to traditional pharmaceuticals
- Advancements in Biotechnology:
- Protein Engineering: Improved methods in protein engineering have enabled the development of more stable and potent biologics.
- Manufacturing Biologics: Advances allow for more efficient production of biologics, still complex and costly as compared to traditional drugs.
- Enhanced Efficacy and Specificity:
Global Small Volume Parenteral Market- Opportunities
- Automation and digitalization: Automation and digitalization are transforming the global small volume parenteral (SVP) market by enhancing manufacturing processes, supply chain management, and overall efficiency. These technologies are becoming increasingly significant due to the precision and safety requirements of parenteral drug production. Here are some key points detailing the impact of automation and digitalization on the SVP market:
- Manufacturing Automation:
- Consistency and Quality: Automated systems ensure consistent quality and compliance with stringent regulatory standards.
- Reduced Contamination Risk: Automation minimizes human intervention, which lowers the risk of contamination—a critical factor in parenteral drug production.
- Scalability: Automated production lines can be scaled up to meet increasing demand without a proportional increase in labor costs.
- Efficiency: Faster and more reliable production processes with fewer errors and less wastage
- Robotic Systems:
- Precision Handling: Robots can handle small volume parenteral more precisely, which is essential for maintaining the integrity of these products.
- Flexibility: Robotic systems can easily be reprogrammed for different tasks, thereby making them well-suited for the SVP market where production batches may be smaller due to the high value and lower volume nature of the products.
- Manufacturing Automation:
- Rising healthcare expenditure: One of the significant factors influencing the growth rate of the global small volume parenteral market is the growing healthcare expenditure, which helps in improving its infrastructure. For instance, according to the International Health Care System of the U.S., in June 2020, U.S. government organizations aim to improve the healthcare infrastructure by increasing funding, setting legislation, and national strategies, and cofounding and setting basic requirements and regulations for the Medicaid program. Similarly, in November 2022, the Canadian Institute for Health Information reported that the total healthcare expenditure in Canada was US$ 331 billion in 2022, or US$ 8,563 per Canadian, while health expenditure represented 12.2% of Canada's gross domestic product (GDP) in 2022, following a high of 13.8% in 2020.
- Advances in healthcare technology: Rapid advances are being made in healthcare delivery approaches to improve patient outcomes. The small volume parenteral market is being positively impacted by the advent of novel technologies that enhance parental drug administration. For example, smart pump intravenous infusion systems with integrated drug libraries and dose error reduction software are being increasingly adopted in hospitals. These smart pumps can be programmed to deliver precise doses of medications based on patient characteristics like weight and medical condition. They also minimize medication errors through safety checks. Ambulatory infusion pumps that are compact and portable have also expanded the utilization of parenteral therapies in home care settings. The development of advanced polymer microsphere and other prolonged release technologies have increased the usage of small volume parenteral formulations for long-acting depot injections. Continuous innovation in drug delivery devices is not only improving medication management but also enabling the shift of care from hospitals to alternate sites like clinics and homes. This expanding access to parenteral therapies presents lucrative growth opportunities for players in the small volume parenteral market.
Global Small Volume Parenteral Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 207.57 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.5% | 2032 Value Projection: | USD 344.37 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Fresenius Kabi AG, Baxter, Sichuan Kelun, BML Parenteral Drugs, ICU Medical, Inc., Pfizer Inc., Becton Dickinson and Company, B. Braun Melsungen AG, WuXi AppTec , Akums Drugs & Pharmaceuticals Ltd., Orion Corporation, Rusoma Laboratories Private Limited, Higgs Healthcare and Syntegon Technology GmbH |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Small Volume Parenteral Market- Trends
- Increasing demand for prefilled syringes: The demand for prefilled syringes in the global small volume parenteral (SVP) market has been steadily increasing and is expected to continue to grow. Prefilled syringes provide several advantages over traditional vial and syringe packaging, which contribute to their rising popularity. Below outlined are the factors driving this demand and potential impacts on the market:
Advantages of Prefilled Syringes:
- Convenience: Prefilled syringes save time and reduce the number of steps needed for medication preparation and administration
- Accuracy: They deliver a precise dose, thus reducing medication errors that are associated with drawing from a vial
- Safety: The closed system of a prefilled syringe reduces the risk of needle-stick injuries and exposure to hazardous drugs
- Stability: Prefilled syringes can enhance the stability of drugs by minimizing their exposure to environmental factors and thereby reducing the risk of contamination.
- Rise in homecare settings: Global small volume parenteral (SVP) market is witnessing a rising trend in homecare settings, which reflects a broader shift towards outpatient care and patient-centric models. The following factors are contributing to this increase:
Growth Drivers for SVP in Homecare Settings:
- Patient Preference: Many patients prefer the comfort and convenience of receiving treatments at home rather than in a clinical setting
- Cost-Effectiveness: Homecare can be more cost-effective for both patients and healthcare systems when compared to prolonged hospital stays
- Technological Advancements: Developments in drug delivery systems, such as prefilled syringes and auto injectors, have made it easier for patients to self-administer parenteral medications safely
- Chronic Disease Management: Prevalence of chronic diseases that require regular parenteral administration of medications, such as diabetes and rheumatoid arthritis, is increasing
Global Small Volume Parenteral Market - Restraints
- Shortage of skilled professionals: The shortage of skilled professionals is a significant challenge impacting the global small volume parenteral (SVP) market. Here are some insights into the issue and possible strategies for addressing the problem:
Factors Contributing to the Shortage:
- Specialized Knowledge: The production, handling, and administration of SVPs require specialized knowledge and expertise that is not as widely available in the workforce
- Growing Market Demand: As the market for SVPs grows, driven by factors such as the rise of biologics and homecare settings, the demand for skilled professionals outpaces the supply.
- High Regulatory Standards: The need for compliance with stringent regulatory standards increases the demand for professionals who are well-versed in good manufacturing practices and regulatory requirements.
Counterbalance: Efficient workforce should be employed who will be capable enough in production, handling and administration of the small volume parenteral market.
- Stringent regulations: Stringent regulations in the global small volume parenteral (SVP) market are pivotal for ensuring patient safety, product quality, and efficacy. These regulations impact various aspects of the market, from product development to distribution. Understanding the regulatory landscape is crucial for companies operating in this space. Here's an outline of how stringent regulations affect the market:
Impact of Stringent Regulations:
- Product Development: Each stage of SVP product development, from formulation to clinical trials, must adhere to strict regulatory guidelines, which can increase the time and cost of bringing new products to market
- Manufacturing and Quality Assurance: Manufacturers must comply with Good Manufacturing Practices (GMP), and products must meet rigorous standards for sterility, stability, and purity. Compliance adds complexity and cost to the production process
- Packaging and Labeling: SVPs require specialized packaging to maintain product integrity, which must meet regulatory standards. Labeling needs to accurately reflect contents and provide clear instructions to ensure safe use.
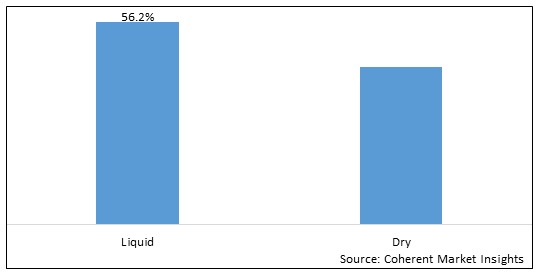
Figure 2. Global Small Volume Parenteral Market Share (%), By Form, 2025
To learn more about this report, Download Free Sample
Global Small Volume Parenteral Market- Recent Developments
Product Approval and Launch
- On September 6, 2023, Amneal Pharmaceuticals, Inc., a pharmaceutical company, announced that they had received Abbreviated New Drug Application (“ANDA”) approval from the U.S. Food and Drug Administration (“FDA”) for calcium gluconate in sodium chloride injection, 1000 mg/50 mL and 2000 mg/100 mL. This injectable product is on the U.S. FDA shortage product list. Calcium gluconate in sodium chloride injection is a small volume parenteral bag indicated for the treatment of acute symptomatic hypocalcemia in pediatric and adult patients.
- In May 2021, Dr. Reddy’s Laboratories Ltd., a pharmaceutical company, launched Ertapenem for Injection, 1 g/vial, a therapeutic equivalent generic version of INVANZ (ertapenem for injection) for injection, 1 g/vial approved by the U.S. Food and Drug Administration (U.S.FDA).
Business Development Activities by the Market Players
- In November 2022, WuXi STA, a subsidiary of WuXi AppTec, a pharmaceutical company announced a new parenteral formulation manufacturing line which has started operation at the drug product site in Wuxi city, China. It is the second line opened for parenteral drug product clinical and commercial manufacturing, with an annual capacity of 10 Mn units. This expansion demonstrates the company’s continuous commitment to enhance its injectable drug product platform.
- In March 2021, Syntegon Technology, a processing and packaging technology company, launched new small volume parenteral (SVP) essential, a cost-efficient version of its proven Pharmatec SVP process systems for the production of small-volume liquid pharmaceuticals.
Top Companies in Global Small Volume Parenteral Market
- Fresenius Kabi AG
- Baxter
- Sichuan Kelun
- BML Parenteral Drugs
- ICU Medical, Inc.
- Pfizer Inc.
- Becton Dickinson and Company
- Braun Melsungen AG
- WuXi AppTec
- Akums Drugs & Pharmaceuticals Ltd.
- Orion Corporation
- Rusoma Laboratories Private Limited
- Higgs Healthcare
- Syntegon Technology GmbH
Definition: Small Volume Parenteral (SVP) which is about a category of pharmaceutical products that are delivered parenteral is administered somewhere other than through the digestive tract, often through injection. SVP usually come in doses of 100 milliliters or less and can encompass a wide range of medications and treatments, including vaccines, medications for chronic conditions, biologics, and other specialized drugs that require parenteral delivery due to their pharmacokinetic properties.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




