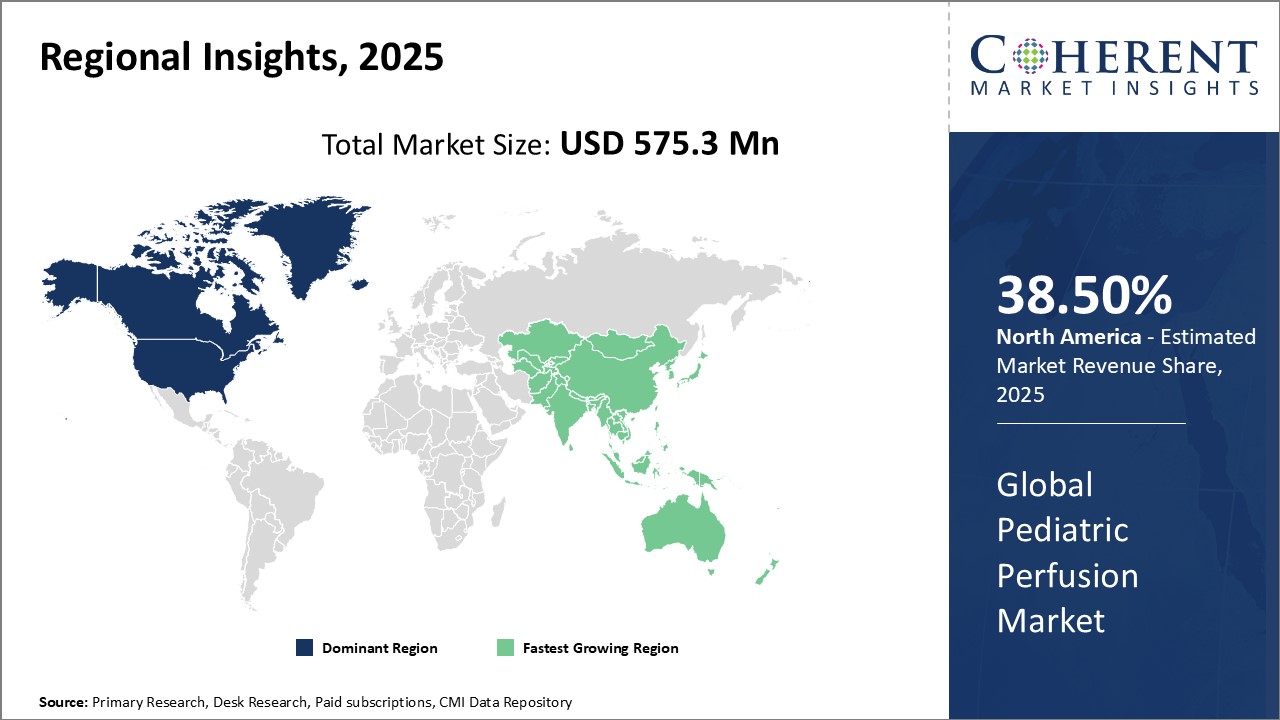
The global pediatric perfusion market size was valued at US$ 575.3 Mn in 2025 and is expected to reach US$ 842.5 Mn by 2032 growing at a compound annual growth rate (CAGR) of 5.6% from 2025 to 2032.
Global Pediatric Perfusion Market Regional Insights:
- North America has established itself as the dominant region in the global pediatric perfusion market. The region has a well-established healthcare infrastructure and high healthcare expenditure. Several leading medical device companies are based in the U.S. and Canada, who invest heavily in R&D to innovate new pediatric perfusion technologies. Hospitals in the region are well-equipped with advanced cardiac facilities and expert perfusionist staff to efficiently conduct pediatric heart surgeries, driving the need for pediatric perfusion systems. Growing awareness about congenital heart diseases and rising pediatric cardiac treatments have boosted the market growth. Moreover, the availability of private and public health insurance has made life-saving pediatric cardiac procedures more accessible.
- Asia Pacific has emerged as the fastest-growing regional market for pediatric perfusion. The Asia Pacific region is projected to be the fastest growing market for pediatric perfusion during the forecast period. Rapid economic growth, improving healthcare infrastructure, and rising medical tourism in countries such as China, India, and Japan are fueling the demand. There is an increase in the incidence of treatable congenital heart diseases due to better diagnosis and screening. This is encouraging hospitals to strengthen their pediatric cardiology departments with advanced equipment such as pediatric heart-lung machines. Also, increased government funding for pediatric healthcare and initiatives to spread awareness will support market expansion. Being a major manufacturing hub, several global Original Equipment Manufacturer (OEM have set up production bases in the region to cater to the rising needs, providing impetus to domestic companies as well. Although import duties remain high on certain medical devices, local companies are striving to indigenously develop affordable pediatric perfusion products. The growing pediatric population base presents lucrative opportunities for both international and domestic players in the pediatric perfusion market.
Analyst View: The pediatric perfusion market is expected to witness steady growth over the next few years. The rising prevalence of congenital heart diseases among children is a major growth driver. Improving healthcare infrastructure and growing awareness about pediatric cardiac treatments are further fueling demand. Advanced perfusion systems offering enhanced safety and efficacy are gaining adoption. However, high costs associated with pediatric cardiac procedures can hamper market growth to an extent. North America currently dominates the pediatric perfusion market and is expected to maintain its leading position. This can be attributed to the presence of advanced healthcare facilities, supportive reimbursement structure, and growing awareness levels in the region. Asia Pacific is projected to emerge as the fastest growing regional market over the forecast period. Increasing medical tourism, rising healthcare expenditures, and growing incidences of congenital heart diseases are favoring market expansion in Asia Pacific. On the product front, heart-lung machines are likely to remain the largest revenue generator owing to wide utilization in various cardiac procedures. However, oxygenators are expected to witness fastest growth on account of the introduction of advanced products with enhanced safety and performance features. Non-facility-based perfusion services are gaining popularity and offering new opportunities for market players. Collaborations aimed at the development of compact and affordable pediatric perfusion systems also present avenues for growth.
Figure 1. Global Pediatric Perfusion Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Pediatric Perfusion Market Drivers:
The increasing incidence of cardiovascular diseases (CVDs) in the pediatric population: The increasing incidence of cardiovascular diseases (CVDs) in the pediatric population is indeed a driving force behind the growth of the pediatric perfusion market. With the rise in congenital heart defects, cardiomyopathies, and other cardiac conditions affecting children, there is a growing demand for expert cardiopulmonary support during surgical interventions. The rise in cardiac conditions necessitates a higher volume of surgical procedures, directly increasing the demand for perfusion technologies. Increasing complexity of congenital heart surgeries requires advanced perfusion techniques and equipment, fueling market innovation and growth. Advancements in pediatric perfusion contribute to higher success rates of cardiac surgeries, garnering trust among healthcare professionals and encouraging the uptake of these procedures. The specific physiological parameters of children require specialized perfusion systems, creating a niche market for tailored solutions. Improvements in the early diagnosis of cardiovascular conditions have led to a broader patient base seeking treatment at earlier stages, augmenting the demand for pediatric perfusion services. Public health efforts to address the burden of pediatric cardiovascular disease may include equipping hospitals with the necessary technologies, such as perfusion systems, which supports market growth. Players in the market can leverage this trend by focusing on developing and providing pediatric-specific perfusion equipment and disposables, investing in training and education for health professionals, and engaging in research and development to improve the safety and effectiveness of perfusion in pediatric cardiac care. For instance, in January 2024, according to data provided by Indian Academy of Pediatrics, congenital heart disease (CHD) is the most frequently occurring congenital disorder, responsible for 28% of all congenital birth defects. The birth prevalence of CHD was reported to be 8-12/1,000 live births in 2018. Considering a rate of 9/1,000, about 1.35 million babies are born with CHD each year globally.
Increasing government initiatives and funding: Increasing government initiatives and funding are indeed pivotal factors that are expected to drive growth in the pediatric perfusion market. As governments around the world recognize the importance of enhancing pediatric cardiac care, there is an increasing allocation of resources and implementation of policies to support the healthcare needs of children with cardiovascular conditions. Government funding often goes towards building and upgrading healthcare facilities, including those required for complex surgeries that necessitate pediatric perfusion, such as heart-lung machines and other supporting equipment. Initiatives that provide financial aid or reimbursement for pediatric surgeries can make these life-saving procedures more accessible and affordable for a wider population. Funding and government-led programs aimed at educating and training medical professionals, including pediatric perfusionists, can expand the workforce capable of delivering high-quality care. Government grants for research can stimulate innovation in the pediatric perfusion field, leading to the development of new and improved technologies that enhance surgical outcomes. Awareness campaigns funded by governments can increase the visibility of pediatric cardiovascular issues and the treatments available, encouraging early diagnosis and treatment. Streamlining regulatory processes for the approval of new medical devices can expedite the availability of advanced pediatric perfusion equipment in the market. Government initiatives that foster collaboration can lead to shared knowledge and resources, further promoting the growth of sophisticated healthcare services like pediatric perfusion. In essence, proactive government involvement can significantly reduce the barriers to quality pediatric cardiac care, directly influencing the scope and scale of the pediatric perfusion market. For market participants, staying abreast of such initiatives and engaging with public entities can be crucial for aligning their business strategies with the evolving landscape.
The emergence of new technologies in pediatric perfusion: The emergence of recent technology in pediatric perfusion is certainly a massive driving force for the pediatric perfusion market. As technological improvements evolve, they create approximately improvements in perfusion system and strategies which are higher ideal for the particular physiological necessities of babies and kids present process cardiac surgery. Development of smaller perfusion circuits and additives designed especially for pediatric patients, decreasing blood product utilization and minimizing the inflammatory reaction frequently visible with large circuits. Enhanced monitoring and control systems in heart-lung machines that allow for more precise management of delicate pediatric perfusion cases result in better patient outcomes. The introduction of safer materials with superior biocompatibility reduce the risk of adverse reactions and improve long-term outcomes for pediatric patients. Use of smart sensors and real-time data analytics to closely monitor patient parameters and perfusion status provide vital information to the perfusionists and surgical team. Advancements in oxygenator and filter technology enhance gas exchange and filtration efficiency, critical for supporting small patients with limited tolerance for physiological fluctuations. Incorporation of robotic elements and automated processes to assist in repetitive or complex tasks enhances precision and reliability of the perfusion process. Technologies aimed at reducing postoperative complications and improving overall outcomes can increase the success rate of pediatric cardiac surgeries and encourage their utilization. The continued evolution of these technologies is fostering a more dynamic and capable pediatric perfusion market. They also open doors for market expansion as improved technologies can mitigate previous risks associated with pediatric perfusion, making surgery a viable option for a broader range of cases. Market players can capitalize on this trend by investing in R&D, collaborating with healthcare professionals to understand clinical needs, and focusing on innovation to meet the stringent demands of pediatric perfusion.
Technological advancements: Technological advancements are indeed a potent catalyst propelling the pediatric perfusion market forward. As technology in healthcare continues to progress, the capabilities of perfusion systems are enhanced, particularly in the context of pediatric care. Innovations in this field are critical because children, especially neonates and infants, present unique challenges due to their smaller anatomical size and different physiological responses compared to adults. Devices specifically designed to accommodate the limited blood volume of pediatric patients reduce the risk of hemodilution and blood transfusions, which is especially important in newborns and small children. Technological improvements often come with better safety features, reducing the risk of complications such as air embolism and blood trauma during surgery. The integration of advanced sensors and monitoring systems allows for more precise management of vital parameters during pediatric cardiac surgeries, improving outcomes and patient safety. Advances in materials used for perfusion equipment can minimize the body's inflammatory response and enhance biocompatibility, which is crucial for the sensitive immune systems of children. Automated perfusion systems can standardize procedures to a certain extent, potentially reducing the variability that comes with manual operations and improving overall results. Software for planning and simulating surgeries can help in individualizing perfusion strategies for pediatric patients, leading to better-prepared surgeries with optimized results. The use of 3D printing technology in creating customized components of perfusion systems allows for better fit and customization, which can be particularly advantageous for pediatric patients. These advancements are bolstering the pediatric perfusion market by providing more effective, safer, and tailored solutions for pediatric cardiac surgery.
Global Pediatric Perfusion Market Opportunities:
- Innovation in perfusion disposables and equipment: Innovation in perfusion disposables and equipment does indeed present a significant opportunity for the pediatric perfusion market. Advancements in these areas can enhance the efficacy, safety, and overall outcomes of pediatric cardiopulmonary bypass procedures, which are essential when performing complex cardiac surgeries in young patients. New designs and materials can minimize the risk of complications associated with perfusion, such as circuit clotting or hemolysis, which is particularly critical for pediatric patients due to their smaller size and unique physiology. Innovations in oxygenators, pumps, and cannula can lead to better oxygen delivery and perfusion efficiency, protecting delicate tissues in pediatric patients during surgery. The development of miniaturized and pediatric-specific equipment addresses the challenge of adapting adult perfusion technology to the needs of infants and children. Advanced equipment with integrated sensors and monitoring capabilities allows for real-time tracking of vital patient parameters, facilitating immediate adjustments during surgery. Single-use, sterile perfusion circuits and disposables can prevent cross-contamination and improve the standardization of care across different healthcare settings. Innovations aimed at reducing the cost of disposables and equipment without compromising quality can make pediatric cardiac surgery more accessible and affordable. Equipment and disposables that can be customized for individual patient needs enable more tailored and effective perfusion strategies, which can be appealing to surgeons. Manufacturers that innovate in disposables and equipment can differentiate their products in the market, capturing the attention of healthcare providers and increasing their market share. The companies that capitalize on these opportunities may foster a competitive edge by meeting the growing demands of the market, aligning with the healthcare industry's push for better, more efficient, and safer medical technologies
- The expansion of application areas for pediatric perfusion: The expansion of application areas for pediatric perfusion presents significant growth opportunities within the market. Pediatric perfusion isn't limited solely to supporting cardiac surgeries; technological advancements and increased understanding of pediatric physiology have opened avenues for its role in other medical situations. Perfusion techniques are becoming increasingly important in pediatric organ transplants, not just preserving organs but also optimizing their condition before transplantation. ECMO is a form of longer-term perfusion support used in pediatric patients with severe heart or lung failure. It has wide-ranging applications, including support during recovery from illnesses, bridge to transplant, or during cardiopulmonary resuscitation (CPR). There is a growing interest in using localized perfusion techniques for delivering high-dose chemotherapy to limbs or organs affected by tumors, minimizing systemic toxicity. Perfusion technologies aid in the management of critically ill neonates and children who need complex fluid and medication management, offering more precise control of their critical physiological needs. Perfusion is also utilized in research, especially in pharmacological studies that require accurate simulation of the human circulatory system. Children born with complex congenital defects may require intervention using perfusion techniques other than traditional surgery, such as in conditions where continuous circulatory or respiratory support is required. In certain complex non-cardiac surgeries where there is a risk of significant blood loss or a need for circulatory support, perfusion technology can be used effectively. The broadening scope of pediatric perfusion applications not only leads to market growth but promotes innovation in perfusion technology, equipment, and disposables designed specifically for the pediatric population. This necessitates the development of new protocols and training programs to ensure safe and effective treatment across these varied applications. For stakeholders in the pediatric perfusion market, diversifying the application areas presents a strategic opportunity to capture new market segments and emphasize the critical role of perfusion in pediatric care.
Global Pediatric Perfusion Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 575.3 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.6% | 2032 Value Projection: | USD 842.5 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, GE Healthcare, LivaNova, Inc., Getinge, Braile Biomedica, XENIOS AG, BL Lifesciences, Terumo Corporation, 3M, Koninklijke Philips N.V., EUROSETS, Merck KGaA, Stryker, Cardinal Health, Nonin, and Sorin Group USA, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Pediatric Perfusion Market Trends:
- Adoption of hybrid perfusion techniques: The adoption of hybrid perfusion techniques is indeed a prominent trend in the pediatric perfusion market. Hybrid perfusion incorporates both conventional cardiopulmonary bypass (CPB) and minimally invasive surgery with the goal of reducing the invasiveness of procedures while maintaining the quality of surgical outcomes. Hybrid techniques, by their nature, are less traumatic for the pediatric patient as they involve smaller incisions and less manipulation of healthy tissue. Smaller incisions typically mean reduced postoperative pain, shorter hospital stays, and quicker overall recovery times for young patients. With reduced exposure of blood to the surfaces of surgical equipment, there is a potential decrease in the systemic inflammatory response, which can lead to fewer complications. The precision of minimally invasive methods, when combined with the stability provided by CPB, can lead to better long-term outcomes for pediatric patients. Given the advantages, there is a growing demand from both parents and healthcare providers for hybrid procedures, which could lead to an increased uptake of specialized perfusion equipment. The trend has been facilitated by advancements in surgical instruments, imaging, and perfusion technology, allowing complex cardiac anomalies to be corrected with precision. Despite potentially higher initial costs for equipment and training, hybrid techniques may be more cost-effective in the long run due to reduced postoperative care requirements and complications. Providers that offer hybrid perfusion services can differentiate themselves in a competitive marketplace, potentially increasing their market share. As the healthcare market continues to evolve, the trend towards hybrid perfusion techniques in pediatric surgery presents a prime opportunity for innovation and growth. For players in the pediatric perfusion market, developing and marketing products that support hybrid techniques could be a strategic move to capitalize on this growing trend.
- Customized pediatric perfusion solutions: Customized pediatric perfusion solutions represent a key trend in the pediatric perfusion market, as they are designed to address the specific needs of infants and children undergoing cardiac surgery. This trend is driven by the understanding that pediatric patients have distinct anatomical and physiological requirements compared to adults, necessitating tailored perfusion strategies. Customization allows for greater precision in perfusion management, potentially resulting in improved surgical outcomes and lower risk of complications for pediatric patients. The need for customization propels innovation in perfusion technologies with advancements like miniaturized circuit components, specialized cannula, and pediatric oxygenators. Preoperative imaging and computer simulations enable the design of perfusion strategies that are individualized based on the patient's cardiac anatomy and physiology, leading to more efficient procedures. Development of perfusion equipment specifically for the pediatric market, such as scaled-down pumps and oxygenators, meets the demand for customization. As awareness of the benefits of customized pediatric perfusion solutions grows, there's an increasing demand from healthcare providers for products and services that offer these tailored options. Opportunities for close collaboration between medical device manufacturers, perfusionists, and cardiac surgeons to develop and refine solutions that align with clinical needs. Manufacturers that offer a wide range of customization options can differentiate their offerings, gaining a competitive advantage in the market. Customization in the pediatric perfusion market reflects the broader trend in healthcare towards personalized medicine. It signifies a more patient-centric approach, ensuring that the unique needs of pediatric patients are met with the most appropriate medical solutions. For companies in the pediatric perfusion space, staying ahead of this trend means investing in R&D, fostering innovation, and working closely with healthcare providers. As personalized medical care continues to grow in importance, customized pediatric perfusion solutions are likely to see increased adoption and market expansion.
Global Pediatric Perfusion Market Restraints:
- The risks associated with pediatric perfusion procedures: The risks associated with pediatric perfusion procedures can indeed pose a challenge to the growth of the pediatric perfusion market. Pediatric perfusion involves supporting or temporarily replacing the patient's circulatory or respiratory function during cardiac surgery, using a heart-lung machine or other perfusion technology. While these procedures are crucial for treating congenital heart defects and other serious cardiac conditions, they also carry inherent risks that could impact market growth. Pediatric perfusion is a highly specialized field, and the procedures are complex. The margin for error is small given the patient's size and the delicate nature of their physiology. Complications can lead to adverse outcomes, which could deter healthcare providers and patients from opting for such surgeries. Potential risks during pediatric perfusion procedures include blood clots, bleeding, infections, and organ dysfunction. These complications can have long-term consequences for the patient’s quality of life and can be costly to manage. The need for specialized training for perfusionists and surgical teams can limit the number of healthcare professionals capable of performing pediatric perfusion. This could restrict the availability of these services and slow market growth. While technology plays a critical role in pediatric perfusion, equipment faults or malfunctions can have serious implications, leading to patient injury or death. The stress associated with the potential risks of pediatric perfusion can affect family decision-making and may influence some to seek alternative treatments with lower perceived risk profiles. Adverse events can lead to legal challenges, which can increase costs for healthcare providers and device manufacturers, as well as lead to increased regulatory scrutiny. Given the high costs and risks associated with pediatric perfusion procedures, insurers may be hesitant to provide coverage, making them less accessible to patients. To mitigate these challenges and continue market growth, continuous innovation in safer and more reliable perfusion technology is essential. Additionally, improving training programs for perfusionists, enhancing surgical techniques, and offering comprehensive follow-up care can help manage the risks associated with pediatric perfusion procedures.
- The high cost of pediatric perfusion procedures: The high cost of pediatric perfusion procedures is indeed a significant hindrance to the growth of the pediatric perfusion market. The high costs associated with complex cardiac surgeries requiring perfusion may limit access to care for families without adequate insurance coverage or in countries with less developed healthcare systems. In regions where insurance companies provide limited reimbursement for pediatric perfusion procedures, there might be a lower rate of surgeries performed. High expenses can affect hospital decisions regarding resource allocation. Hospitals with limited budgets may prioritize other services over the costly pediatric perfusion procedures. Socioeconomic disparities can exacerbate issues of access, as underprivileged populations may struggle to afford the necessary care, leading to unmet needs and potential market contraction. Healthcare providers may be more hesitant to invest in expensive perfusion systems and the training required to operate them given the financial implications. The financial burden on families considering pediatric cardiac surgery can be substantial, leading to delayed or forgone surgeries, which directly impacts market demand. Larger production volumes can decrease the unit cost of perfusion equipment. Streamlining surgical procedures and hospital stays can reduce overhead costs and make pediatric perfusion more cost-effective. Advocacy for broader insurance coverage and governmental or non-profit subsidies for pediatric cardiac care can alleviate financial barriers to access. Understanding the cost dynamics and developing strategies to mitigate the high expenses associated with pediatric perfusion procedures will be critical in continuing the market's growth trajectory.
Recent Developments:
New Product Launches & Approval:
In March 2023, LivaNova PLC, a medical technology and innovation company, announced that it has received the U.S. Food and Drug Administration (FDA) 510(k) clearance for its Essenz Heart-Lung Machine (HLM). LivaNova PLC initiated the commercial launch of Essenz in the U.S.
In January 2022, XVIVO, a medical technology company, received ‘510(k) clearance’ from the U.S. FDA for its portable organ perfusion system, a Kidney Assist Transport. It facilitates donor kidneys' continuous hypothermic pulsatile perfusion using an oxygenated preservation solution throughout the transportation process from the donor to the recipient in transplant procedures.
In November 2021, Dongguan Kewei Medical Instrument Co., Ltd., a subsidiary of MicroPort Scientific Corporation, a medical device company, received approval of National Medical Products Administration (NMPA) of China for the Vitasprings Spiral Diversion Integrated Membrane Oxygenator. Vitasprings is a product developed in China that imitates the function of the lungs in cardiopulmonary bypass and adopts an innovative hemodynamic structure featuring a spiral flow guide and secondary diversion combined with a vortex exhaust design, which can reduce damage to blood cells.
Acquisition, Collaboration, and Partnerships:
In September 2022, CHS USA Inc., a privately-held medical device manufacturer, announced the acquisition of SandBox Medical LLC, the manufacturer and source for JollyPop Pacifiers and other products designed for premature babies and full-term newborns. This acquisition will increase CHS USA Inc.'s presence in the Neonatal Intensive Care Unit (NICU) and pediatric market, while providing innovative products for premature babies and full-term newborns.
In September 2020, XVIVO, a medical technology company, announced acquisition of Organ Assist B.V., a company that focuses primarily on developing machines and consumables for liver and kidney perfusion. Through the acquisition, XVIVO became the first organ preservation and evaluation company to be actively involved in all major organs, which accelerates the company’s strategy of becoming a global all organ provider.
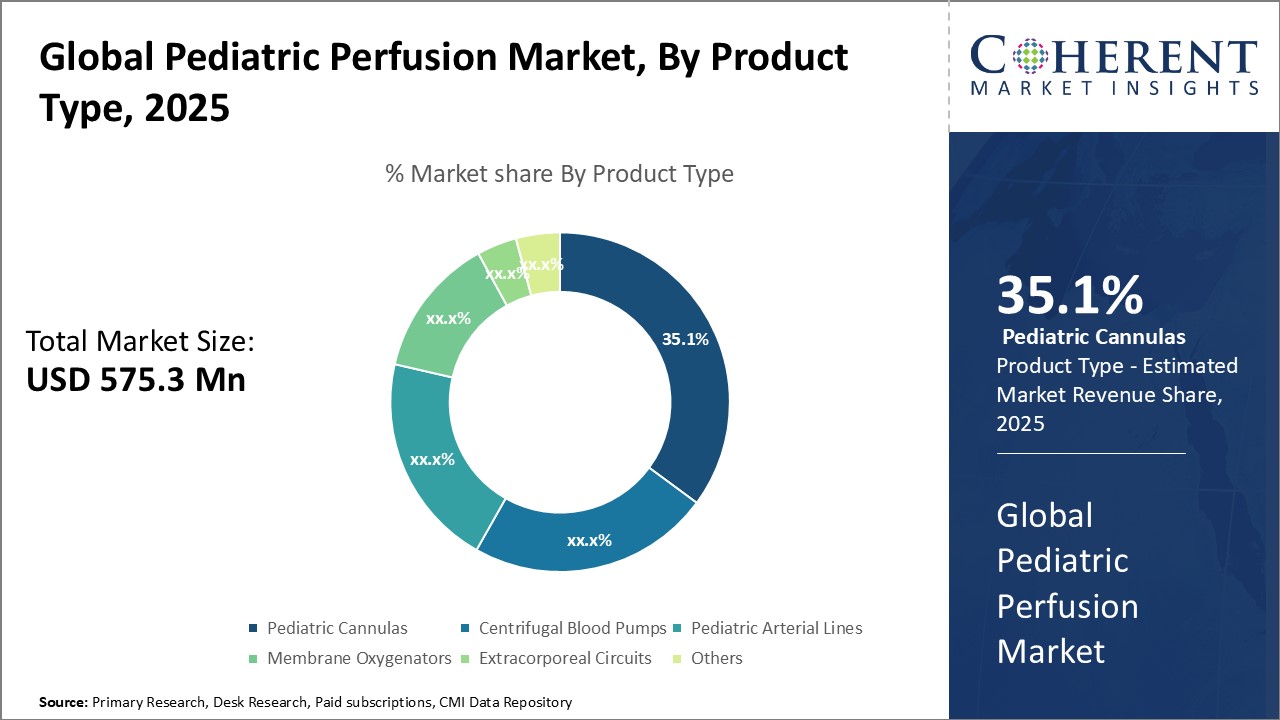
Figure 2. Global Pediatric Perfusion Market Share (%), By Product Type, 2025
To learn more about this report, Download Free Sample
Top Companies in the Global Pediatric Perfusion Market:
- Medtronic
- GE Healthcare
- LivaNova, PLC.
- Getinge
- Braile Biomedica
- XENIOS AG
- BL Lifesciences
- Terumo Corporation
- 3M
- Koninklijke Philips N.V.
- EUROSETS
- Merck KGaA
- Stryker
- Cardinal Health
- Nonin
- Sorin Group USA, Inc.
Definition: Pediatric perfusion refers to the specialized practice of using cardiopulmonary bypass and extracorporeal circulation techniques during cardiac surgery for infants and children. It involves the use of a heart-lung machine and associated equipment to maintain blood flow and oxygenation to the body when the child's heart or lungs are unable to function properly or need to be temporarily stopped during surgery. This practice is crucial in treating congenital heart defects, which are structural heart problems present at birth, as well as other pediatric cardiac conditions requiring surgical intervention. Pediatric perfusionists are skilled professionals trained to handle these sophisticated devices, and they play a critical role in the success of pediatric cardiac surgical procedures. Due to the smaller size and unique physiological characteristics of pediatric patients, the equipment and procedures used in pediatric perfusion are specifically designed to be more precise and gentle compared to adult perfusion techniques. This field demands a high level of expertise due to the complexities and delicacies involved in managing the circulatory needs of children and ensuring their safety throughout the surgical process.
Pediatric perfusion refers to specialized procedures that utilize extracorporeal circulation techniques to oxygenate and circulate blood outside the body during heart surgery in children. There are various perfusion products and equipment required to effectively support the cardiovascular system during such complex surgeries.
The key products used in pediatric perfusion include heart-lung machines, oxygenators, pumps, cannula, and tubing sets. Heart-lung machines integrate a pump and oxygenator to circulate and oxygenate the child's blood outside their body during surgery. Oxygenators infuse oxygen and remove carbon dioxide from blood. Pumps are crucial to drive blood flow through the circuit while cannula provides vascular access points. Tubing sets connect all components to form a fully-integrated perfusion circuit.
Each product type has its advantages but also disadvantages. For example, while heart-lung machines effectively support heart and lung function, they are also large, expensive, and require specialized personnel to operate. Oxygenators efficiently oxygenate blood but surface area limitations may impact gas exchange in small infants. Pumps and cannula introduce foreign surfaces that require careful biocompatibility. The perfusion circuit complexity also introduces risks of air embolism or component malfunction. Advancements in miniaturization and safety features aim to reduce downsides, but pediatric perfusion still requires specialized professional oversight.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




