The global NPHP5 retinal degeneration treatment market size is expected to reach US$ 133.3 Mn by 2032 from US$ 15.8 Mn in 2025, exhibiting a compound annual growth rate (CAGR) of 35.6% during the forecast period.
NPHP5 retinal degeneration is a rare, inherited retinal ciliopathy that causes progressive vision loss. It is caused by mutations in the NPHP5 gene, which helps maintain the structure and function of photoreceptor cilia in the eye.
Global NPHP5 Retinal Degeneration Treatment Market- Regional Insights
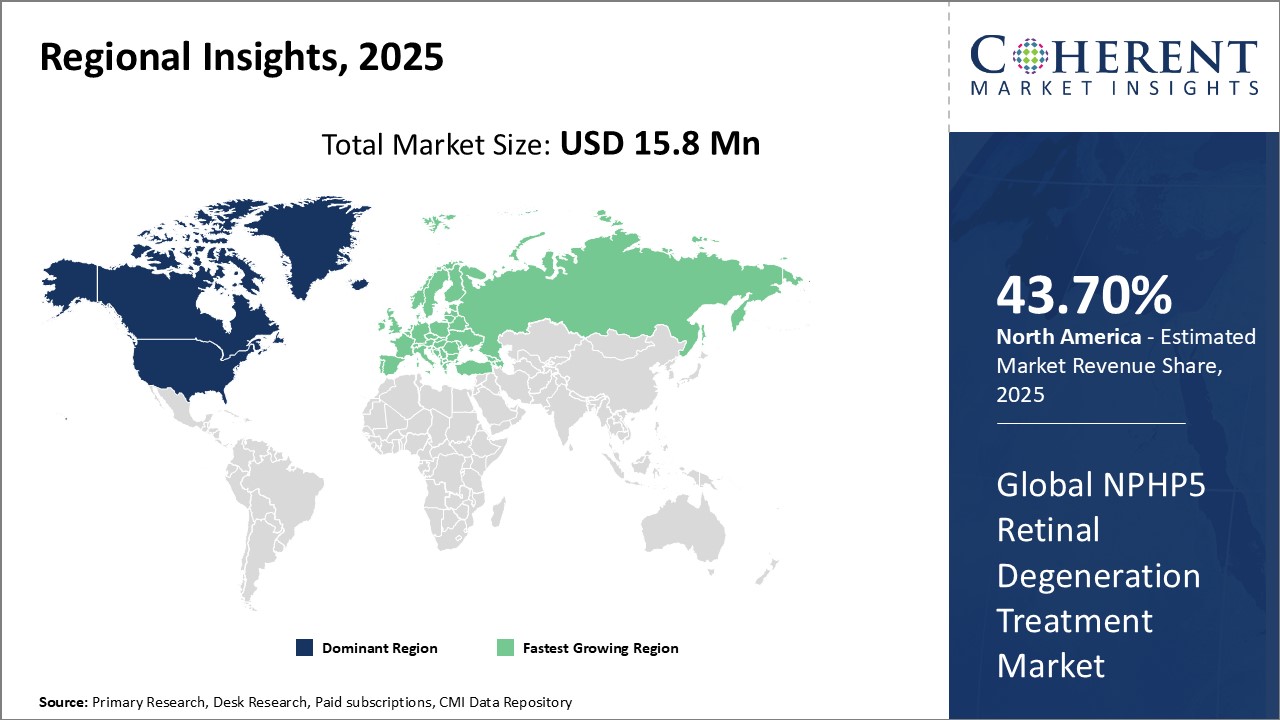
- North America is expected to be the largest market for the NPHP5 retinal degeneration treatment market during the forecast period, accounting for over 43.7% of the market share in 2025. The North American region currently dominates the global NPHP5 retinal degeneration treatment market. With the presence of several top pharmaceutical companies and a large healthcare expenditure in North America.
- Asia Pacific market is expected to be the second-largest market for NPHP5 retinal degeneration treatment market, accounting for over 25.2% of the market share in 2025. The Asia Pacific region, especially emerging countries like China and India, is witnessing the fastest growth in the Chaple disease therapeutics market. This can be attributed to increasing research and development activities, partnerships, and agreements among key market players.
- Europe market is expected to be the fastest-growing market for the NPHP5 retinal degeneration treatment market, with a share of 19% during the forecast period. The growth of the market in Europe is emerging as a hotspot at a rapid pace owing to favorable policy support, infrastructure developments, and evolving customer preferences.
Figure 1. Global NPHP5 Retinal Degeneration Treatment Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Analyst View:
The global NPHP5 retinal degeneration treatment market is expected to witness moderate growth over the forecast period. The increasing prevalence of NPHP5 retinal degeneration disease worldwide will be a key growth driver for this market. Other major factors, such as rising healthcare expenditure in developing nations and growing awareness about available treatment options, will further propel the demand for NPHP5 retinal degeneration treatment drugs.
Global NPHP5 Retinal Degeneration Treatment Market - Drivers
- Increasing Launch of Awareness Campaigns: Increasing adoption of inorganic growth strategies, such as the launch of awareness campaigns, is expected to drive market growth over the forecast period. For instance, in July 2025, the American Society of Retina Specialists (ASRS) announced the launch of an awareness campaign to increase awareness of retinal disease. The aim of the campaign is to educate the public about protecting their vision from preventable blindness and vision loss due to retinal disease and the importance of expert retina specialist care.
- Increasing Investments by Key Market Players: Increasing adoption of inorganic growth strategies such as investments by key market players is expected to drive the market growth over the forecast period. For instance, on October 16, 2025, The Retinal Degeneration Fund, the venture arm of the Foundation Fighting Blindness, announced an investment in NVasc, an early-stage company focused on treating age-related macular degeneration (AMD).
Global NPHP5 Retinal Degeneration Treatment Market - Opportunities
- Emerging markets in Asia Pacific and Latin America: Emerging markets in Asia Pacific and Latin America offer immense potential for growth in the global NPHP5 retinal degeneration treatment market. Retinal degeneration is prevalent in developing nations and according to the World Health Organization, countries like India, Brazil, China, and Mexico account for a major share of the global disease burden. These emerging economies are witnessing rapid economic and infrastructural development, which is improving access to healthcare. Simultaneously, growing health awareness and expanded insurance coverage are enabling more patients to seek treatment. Pharmaceutical companies can capitalize on these favorable dynamics. Governments in the region have indicated their commitment to tackling non-communicable diseases and several have launched public health programs focused on the early detection and management of conditions like Chaple disease. For instance, the National Medical Mission under India's Ayushman Bharat initiative aims to set up 1.5 million health and wellness centers by 2023, which will boost screening efforts (NITI Aayog, 2020).
- Rising healthcare expenditure: The significant factor influencing the growth rate of the global NPHP5 retinal degeneration treatment market is the growing healthcare expenditure, which helps in improving its infrastructure. For instance, according to the International Health Care System of the U.S., in June 2020, U.S. government organizations aim to improve the healthcare infrastructure by increasing funding, setting legislation and national strategies, and cofounding and setting basic requirements and regulations for the Medicaid program. Similarly, in November 2022, the Canadian Institute for Health Information reported that the total healthcare expenditure in Canada was US$ 331 billion in 2022, or US$ 8,563 per Canadian, while health expenditure represented 12.2% of Canada's gross domestic product (GDP) in 2022, following a high of 13.8% in 2020.
Global NPHP5 Retinal Degeneration Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 15.8 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 35.6% | 2032 Value Projection: | USD 133.3 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
ProQR Therapeutics, Editas Medicine, Nanoscope Therapeutics, Inc., jCyte, Inc., Biogen, Novartis AG, Spark Therapeutics, MeiraGTx, NightstaRx, Beacon Therapeutics, Applied Genetic Technologies Corporation, ViGeneron and RetinAI Medical |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global NPHP5 Retinal Degeneration Treatment Market - Trends
- Increasing Partnerships Among Key Market Players: Increasing adoption of inorganic growth strategies such as partnerships among key market players. For instance, on April 26, 2023, RetinAI Medical, a medical technology company, announced a partnership with Boehringer Ingelheim, a global pharmaceutical company, to improve retinal disease treatment. Under the terms of the partnership, RetinAI will test its AI tools by analyzing Boehringer Ingelheim’s imaging datasets from clinical studies and real-world evidence. The aim is to identify novel biomarkers and predictors of disease progression that could accelerate the development of urgently needed treatments.
Global NPHP5 Retinal Degeneration Treatment Market - Restraints
- Long approval timelines: The lengthy approval timelines required for new therapeutic drugs are significantly hampering the growth of the global NPHP5 retinal degeneration treatment market. Obtaining regulatory approvals from entities like the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) is a rigorous, time-consuming and costly process. On average, it takes 8-12 years for a potential new treatment to navigate through the stages of pre-clinical and clinical trials and regulatory review before it can be available for patients. This prolonged timeline poses challenges for biopharmaceutical companies as well as patients. For drug developers, the vast time commitment and investment required to bring a treatment to market affects their return on investment and deters them from pursuing new therapies. It also delays patient access to potentially life-changing or curative innovations. This is especially concerning in the case of rare or orphan diseases, like Chaple disease where the patient pool itself is small. Any delays in therapy availability directly impacts the lives and outcomes of these vulnerable patient populations, who have limited or no treatment options otherwise.
- Counterbalance – Thus, key market players can conduct thorough research, compile all required documentation ahead of time, and ensure all materials submitted for approval meet the necessary guidelines and are as complete as possible.
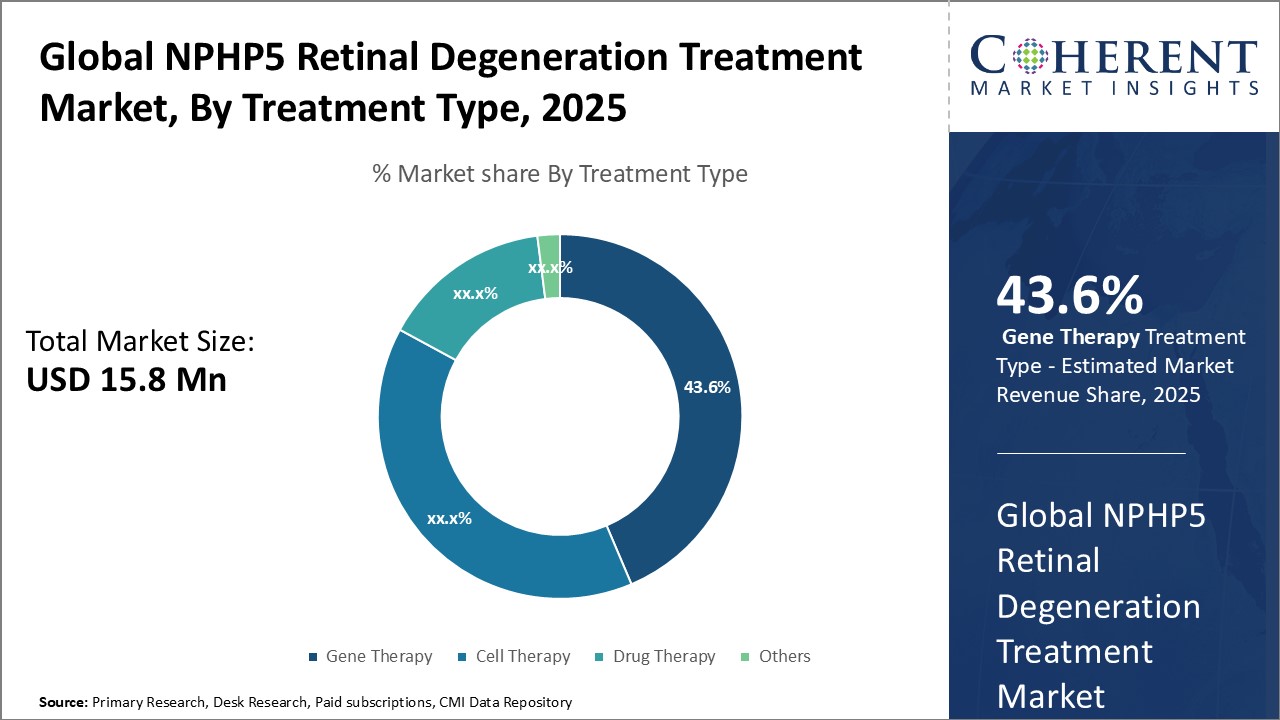
Figure 2. Global NPHP5 Retinal Degeneration Treatment Market Share (%), By Treatment Type, 2025
To learn more about this report, Download Free Sample
Global NPHP5 Retinal Degeneration Treatment Market – Key Developments
- On October 16, 2023, The Retinal Degeneration Fund, the venture arm of the Foundation Fighting Blindness, announced an investment in NVasc, an early-stage company focused on treating age-related macular degeneration (AMD).
- On April 26, 2023, RetinAI Medical, a medical technology company, announced a partnership with Boehringer Ingelheim, a global pharmaceutical company, to improve retinal disease treatment. Under the terms of the partnership, RetinAI will test its AI tools by analyzing Boehringer Ingelheim’s imaging datasets from clinical studies and real-world evidence. The aim is to identify novel biomarkers and predictors of disease progression that could accelerate the development of urgently needed treatments.
Top Companies in Global NPHP5 Retinal Degeneration Treatment Market
- ProQR Therapeutics
- Editas Medicine
- Nanoscope Therapeutics, Inc.
- jCyte, Inc.
- Biogen
- Novartis AG
- Spark Therapeutics
- MeiraGTx
- NightstaRx
- Beacon Therapeutics
- Applied Genetic Technologies Corporation
- ViGeneron
- RetinAI Medical
Definition: NPHP5 retinal degeneration is a rare, inherited retinal ciliopathy that causes progressive vision loss. It is caused by mutations in the NPHP5 gene, which helps maintain the structure and function of photoreceptor cilia in the eye.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




