The global multiplex assay market size is expected to reach US$ 4.45 Bn by 2032, from US$ 1.7 Bn in 2025, at a CAGR of 14.7% during the forecast period.
Multiplex assays allow the detection and quantification of multiple analytes simultaneously in a single reaction volume. There are two main types of multiplex assays - protein-based and nucleic acid-based. Protein-based multiplex assays, such as multiplex Enzyme-Linked Immuno Sorbent Assay (ELISA), allow the testing of multiple protein biomarkers concurrently. They have advantages like high sensitivity and ability to analyze low-volume samples. However, they require specialized instrumentations and consumables which increase costs.
Nucleic acid-based multiplex assays detect and quantify DNA or RNA targets in parallel. Popular nucleic acid-based multiplex techniques include polymerase chain reaction (PCR)-based assays and microarray-based assays. PCR-based multiplex assays can amplify and detect up to 100 DNA targets simultaneously with high precision. This allows analyzing multiple genes related to a disease condition in one reaction, saving time and sample volume compared to single-target assays. However, they are prone to interference between primer sets, limiting the number of targets analyzed jointly. Microarray-based assays can analyze thousands of DNA/RNA targets in a single experiment. Nevertheless, they require specialized equipment and skilled personnel for complex assay procedures. In summary, multiplex assays provide scale and throughput benefits over single-target assays, though intricate assay optimization is needed depending on the platform's limitations.
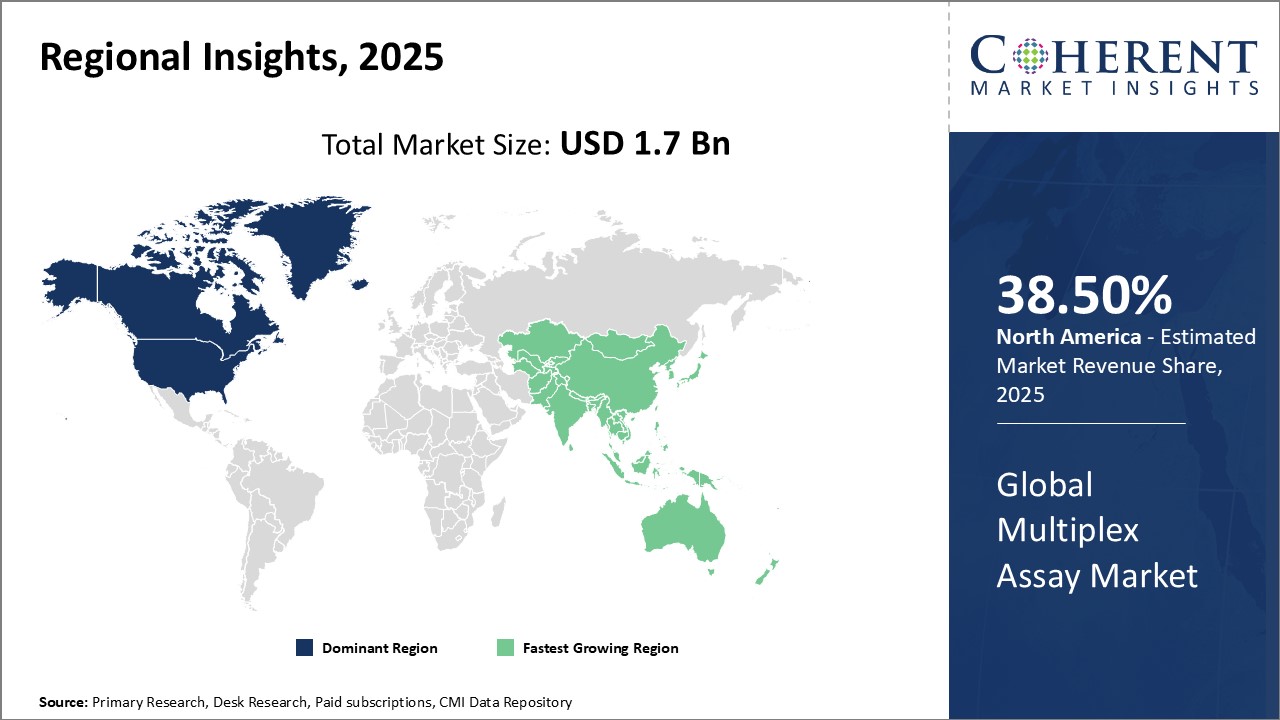
Global Multiplex Assay Market Regional Insights:
- North America has established itself as the dominant region in the global multiplex assay market. This can be attributed to the strong prevalence of chronic and lifestyle diseases in the region coupled with sophisticated healthcare infrastructure and high healthcare spending. Countries like the U.S. and Canada have major pharmaceutical and research companies with a strong focus on precision medicine and companion diagnostics. There is also a significant amount of funding available for research and development from both private and government sources. Furthermore, the presence of top scientific institutions and availability of skilled labor has enabled North America to emerge as a leader in biotechnology and life sciences. Advanced diagnostic technologies are widely adopted in the region and multiplex assays allow the parallel testing of multiple biomarkers, driving their demand. Stringent regulations ensure product quality which has enhanced customer confidence in these assays. With a thriving biopharma industry, original equipment manufacturers find North America as the most lucrative market for their multiplex assay products and solutions.
- Asia Pacific countries like China, India, Japan, and South Korea are experiencing rising income levels and growing health awareness. This has increased the demand for advanced testing capabilities especially as the disease burden of chronic illnesses increases. The multiplex assay market is benefitting from these economic and healthcare trends. Governments in Asia Pacific are focusing on developing world-class medical infrastructure and improving access to healthcare. This has provided opportunities to both international as well as local multiplex assay companies to increase their footprint in the emerging markets. The availability of skilled resources at relatively lower costs makes Asia Pacific an attractive hub for the outsourcing of research activities involving multiplex assays. With enhanced connectivity and adoption of new technologies, the region is projected to experience fastest gains.
Figure 1. Global Multiplex Assay Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Analyst’s Views: The multiplex assay market is poised to grow significantly in the coming years. Major drivers of growth include increasing investments in personalized and precision medicine as multiplex assays allow analyzing many biomarkers simultaneously. This gives valuable insights into disease diagnosis, progression, and personalized treatment options. Additionally, the growing research activity in fields like oncology, immunology, and infectious diseases is also expected to drive the demand for multiplex assays as they offer high-throughput and are cost-effective for clinical trials and research applications. However, stringent regulations for the approval of new diagnostic kits may restrain the market growth to some extent. Furthermore, the requirement of sizable investments and expertise in bioinformatics for assay development and data analysis poses challenges, especially for small and mid-sized players. The North American region currently dominates the market and is expected to continue its dominance during the forecast period. This can be attributed to factors like growing research budgets, well-established biotech industry, and increasing healthcare expenditure. Asia Pacific is projected to experience the highest growth owing to rising healthcare awareness, improving research infrastructure, and growing biotech industry in countries like China and India. Overall, the multiplex assay market has strong growth opportunities during the forecast period with advancements in precision diagnostics, companion diagnostics, and personalized medicine.
Global Multiplex Assay Market Drivers:
- Advancing genomics and proteomics research: The field of genomics and proteomics research has seen tremendous growth over the past few decades. Next-generation sequencing technologies have enabled researchers to more efficiently sequence whole genomes and analyze gene expression patterns. At the same time, technologies for proteomics analysis including mass spectrometry have advanced our understanding of protein structure, function, and interactions. The large volumes of data generated through such research require high-throughput screening methods to validate findings and uncover new insights. Multiplex assays have proven invaluable for researchers as they allow simultaneous testing of multiple biomarker candidates or pathways in a single experiment. This saves precious time and biological sample quantity compared to running individual assays. Leading pharmaceutical and biotechnology companies have significantly ramped up their genomics and proteomics research efforts in pursuit of novel drug targets. Academia too has witnessed a massive increase in the number of genome sequencing and functional genomics projects worldwide. All of this research demands powerful screening tools like multiplex assays to make the most of the data. Their continued adoption will be crucial for furthering our understanding of human health and disease at the molecular level.
- Increased focus on precision medicine: Precision medicine, which tailors medical treatment to the individual characteristics of each patient, has become a major goal for healthcare systems globally. A key tenet of precision medicine is to match patients with the therapies that will work best for them based on specific biomarkers. This requires stratifying patients into relevant sub-groups using diagnostic tests. Multiplex assays have emerged as an important technology for precision medicine as they enable simultaneous testing of multiple biomarkers such as genomic alterations, protein expression levels, and metabolic signatures from a single biological sample. Their high-throughput screening abilities are well-suited for analyzing the complex molecular profiles necessary for precision treatment approaches. Many pharmaceutical companies are engaged in companion diagnostic development, and regulatory agencies now consider biomarker evidence when approving certain drugs. The clinical momentum behind precision medicine and emphasis on companion diagnostics will boost demand for flexible, multiplexed testing solutions in the coming years. As healthcare moves increasingly towards a more personalized paradigm focused on biomarkers and molecular signatures, multiplex assays are poised to play a significant role.
- Increasing product launches: New products may introduce improved technologies or novel applications, which can lead to more efficient assays, enhanced sensitivity, higher throughput, or broader multiplexing capabilities. As more products enter the market, competition increases, which can fuel faster innovation cycles and potentially lead to reductions in costs. Frequent product launches keep customers engaged, as they are continually offered new solutions that may enhance their research or diagnostics capabilities. Products might cater to previously underserved market segments, such as smaller labs that require less complex systems, or highly specialized research fields. By launching products in new geographical markets, companies can expand their global footprint, increasing the accessibility of multiplex assays worldwide. With each new product that gains regulatory approval, the credibility of multiplex assays grows, supporting broader adoption in clinical settings. Product launches often come with a suite of accompanying services, such as customer support and training, which can enhance the user experience and encourage uptake. For instance, in March 2021, Promega Corporation, a biotechnology company, announced the launch of XpressAmp Direct Amplification Reagents that facilitate RNA extraction-free sample preparation that is automation-friendly. With this innovation, laboratories testing for COVID-19 have a new tool that enables them to skip the potentially bottlenecked RNA extraction step of the workflow and move directly to polymerase chain reaction (PCR) amplification.
Global Multiplex Assay Market Opportunities:
- Untapped opportunities in pharmaceutical and biotechnology companies: Multiplex assays can be used in various stages of drug development, from target discovery and validation to toxicity testing and biomarker analysis. This versatility means they can significantly streamline and enhance the drug development process. The need for high-throughput screening in drug discovery is ever-present, and multiplex assays fit well into this space, allowing simultaneous testing of multiple drug candidates against a range of targets. There is an ongoing search for new biomarkers that can predict drug efficacy and patient response. Multiplex assays allow the simultaneous measurement of various potential biomarkers, speeding up the discovery process. The push towards personalized medicine in both pharmaceuticals and biotech relies heavily on the ability to profile multiple biological markers to tailor treatments to individual patients. Multiplex assays are integral to this process. In clinical trials, multiplex assays can be used for patient stratification and to monitor responses to treatment, providing a more detailed and comprehensive analysis than single-assay methods. Biotechnology companies, particularly those involved in the manufacture of biological products, require stringent quality control measures, and multiplex assays can monitor multiple quality attributes at once.
- Incorporation of multiplex assay in clinical trials: Multiplex assays enable simultaneous monitoring of multiple biomarkers, providing comprehensive data on drug efficacy and patient safety. They can identify patient subgroups based on biomarker profiles, which can lead to more personalized and potentially effective treatments. By providing robust data, multiplex assays can aid in making faster decisions during the drug development process, potentially shortening clinical trial durations and reducing costs. Multiplex assays can assist in predicting treatment response, thus improving the design and outcome of clinical trials. Assays developed alongside therapeutics can serve as companion diagnostics to determine the appropriate use of a specific drug in targeted populations. Data from multiplex assays can support regulatory submissions by providing detailed information on drug action and patient response. As they can analyze multiple targets in a single run, multiplex assays are cost-effective, requiring fewer samples and less labor.
Global Multiplex Assay Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.7 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 14.7% | 2032 Value Projection: | USD 4.45 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Luminex Corporation, Thermo Fisher Scientific, Inc., Illumina, Inc., Bio-Rad Laboratories, Inc., Qiagen, Abcam Plc., Becton, Dickinson and Company, Merck KGaA, Agilent Technologies, MESO SCALE DIAGNOSTICS, LLC., Randox Laboratories Ltd., Seegene Inc, Perkin Elmer Inc., R&D Systems, Inc., and Advanced Cell Diagnostics, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Multiplex Assay Market Trends:
- Adoption of automated multiplex assay systems: Automated systems can process multiple samples simultaneously and more quickly than manual methods, significantly increasing throughput and productivity. Automation ensures that each step of the assay is performed consistently, improving the standardization and reproducibility of results, which is especially important in clinical settings and large-scale studies. Automated systems can reduce the risk of error in sample handling and data recording, leading to higher quality data. In the long term, automated systems can be more cost-effective by reducing labor costs and minimizing the potential for costly mistakes. These systems often feature user-friendly interfaces and software that simplify the multiplex assay setup and analysis, making them accessible to a broader range of users. Automated systems can be integrated with existing laboratory information management systems (LIMS), facilitating seamless data transfer and management. Modern automated systems come equipped with advanced optics and detection technologies that improve the sensitivity and dynamic range of the assays. Automated systems often offer modularity and scalability, allowing laboratories to adjust the capacity of their multiplex assay systems based on current demands. By minimizing the need for direct human handling of samples and reagents, automated systems can enhance laboratory safety. Automated systems support the execution of more complex multiplex assays that might be challenging to perform manually, expanding the possibilities for multi-parametric analysis.
- The emergence of cloud-based multiplex assay platforms: Cloud-based systems enable efficient handling of the extensive data generated by multiplex assays, facilitating better data storage, retrieval, and analysis without the need for substantial physical IT infrastructure. As demand fluctuates, cloud services can be easily scaled up or down, allowing laboratories to adjust their computational resources according to their current needs without significant capital investment. Cloud platforms can dramatically enhance collaboration between researchers and institutions, enabling seamless data sharing and analysis across multiple locations. By using cloud-based platforms, users can access their data and assay tools from anywhere, at any time, using any compatible device with internet connectivity. Cloud-based multiplex platforms can be integrated with other laboratory information systems (LIS) and electronic laboratory notebooks (ELNs), streamlining workflows and ensuring that data is easily transferrable between different systems and stages of the research and development process. The cloud can facilitate real-time data analysis, which can be particularly useful for time-sensitive applications, such as infectious disease surveillance or monitoring of clinical trials. For many labs, especially smaller ones or those in startup phases, cloud-based solutions can be more cost-effective, reducing the need for investment in expensive servers and IT maintenance.
Global Multiplex Assay Market Restraints:
- Stringent government regulations: Gaining approval for multiplex assays can be complex due to the need to validate multiple analytes simultaneously. Regulatory bodies such as the FDA or EMA require robust evidence to demonstrate the efficacy and safety of such assays. The process can be lengthy and resource-intensive. Maintaining consistent quality control across multiple analytes within a single assay can be challenging, and any variance or inconsistency may raise concerns from regulatory agencies. Multiplex assays generate a large amount of data, and regulatory bodies may require detailed evidence to ensure that the data can be interpreted accurately and reliably. The lack of harmonized standards for multiplex assays can be a hurdle, as different jurisdictions may have varying requirements for assay performance and validation. Even if regulatory approval is obtained, reimbursement hurdles may persist. Payers may require additional evidence of clinical utility and cost-effectiveness before covering multiplex assay tests. Specific challenges arise when multiplex assays are used as companion diagnostics. Regulatory bodies must ensure that the assays are accurately identifying patients who will benefit from a particular therapeutic intervention. The regulatory landscape for multiplex assays can evolve, with the introduction of new guidelines or amendments to existing ones. Companies must stay up to date and compliant with these changes, which can incur additional costs and delays. Companies seeking to market their multiplex assays internationally face the complexity of navigating different regulatory requirements across different countries or regions. As a market research analyst, it is important to continuously monitor these regulatory issues and assess their impact on market dynamics. This includes keeping track of guidance documents, regulatory approvals, and policy shifts. Companies will need to closely engage with regulatory experts to navigate the approval process successfully and address any potential issues proactively
- High capital investment required: The cost of purchasing or upgrading to sophisticated instruments capable of performing multiplex assays can be substantial, potentially limiting the ability of smaller laboratories or research institutions to adopt this technology. These systems often require ongoing maintenance as well as skilled personnel to operate them, adding to the long-term operational costs. Sophisticated software is required to analyze and interpret the complex data generated by multiplex assays. The expense of purchasing, licensing, and updating this software can be considerable. Laboratories must invest in training their staff to use multiplex assay platforms properly, which can be resource-intensive. Meeting strict regulatory requirements for multiplex assays, particularly those used in clinical diagnostics, can incur additional costs in terms of validation, documentation, and quality control processes. With rapidly advancing technology, instrumentation can quickly become outdated, leading to further investment to stay current with the latest capabilities for New High startup costs can deter new companies from entering the multiplex assay market, potentially limiting competition and innovation. To mitigate these concerns, it is essential to recommend strategies for cost management such as leasing equipment or using cloud-based software platforms that offer pay-as-you-go pricing models.
Recent Developments:
New product launches & approvals:
In May 2022, Vela Diagnostics, a biotechnology company, announced the launch of PathoKey MP UTI ID and AMR PCR Test for research use only. This multiplex PCR-based test allows for the in-vitro detection and differentiation of 14 pathogens that cause urinary tract infections (UTI), as well as 14 antimicrobial resistance (AMR) genes encoding resistance to five antibiotics.
In May 2022, Sansure Biotech Inc., a biotechnology company, announced the launch of the Six Respiratory Pathogens Multiplex Nucleic Acid Diagnostic Kit (Multiplex PCR-Fluorescence Probing) developed by Sansure Biotech Inc., was approved for marketing (GXZZ 20223400597). It is the first domestic test reagent based on multiplex fluorescence PCR technology to detect six common bacteria in a single tube.
In November 2020, QIAGEN, a biotechnology company, announced the European launch of the NeuMoDxFlu A-B/RSV/SARS-CoV-2 Vantage Test that will help healthcare professionals quickly identify and differentiate between patients with common seasonal respiratory infections and COVID-19. With the Northern Hemisphere in the grip of flu season, this multiplex polymerase chain reaction (PCR) test detects and differentiates influenzas A and B, respiratory syncytial virus (RSV) and SARS-CoV-2 infections within 80 minutes. These viruses produce similar respiratory symptoms, making it essential to provide differential diagnosis among them for patient treatment and management decisions, especially during the COVID-19 pandemic.
Acquisition, collaboration and partnerships:
In June 2023, Bio-Techne Corporation, a global life sciences company, acquired Lunaphore, Inc., a leading developer of fully automated spatial biology solutions, to support the development of diagnostic tools and streamline clinical trials, and ultimately improve patient outcomes
In May 2022, CellCarta, a leading global provider of precision medicine laboratory services, announced the acquisition of the commercial rights to the antibody panels and assays from Precision Assays, a leader in next-generation targeted proteomics testing solutions. Precision Assays develops and deploys high-end multiplex quantitative immuno-MRM mass spectrophotometry-based assays for its pharmaceutical and biotech industry clients.
In April 2021, DiaSorin S.p.A., a global leader in the In Vitro Diagnostic (IVD) field, announced the acquisition of Luminex Corporation, a leader in multiplexing technology. The acquisition will broaden DiaSorin S.p.A.,'s positioning in the molecular diagnostics space and strengthen its existing value proposition in line with its strategic priorities. Through this acquisition, DiaSorin S.p.A gained access to Luminex Corporation’s molecular diagnostics multiplexing technology and a portfolio that will strengthen its existing offering while expanding its presence in the U.S.
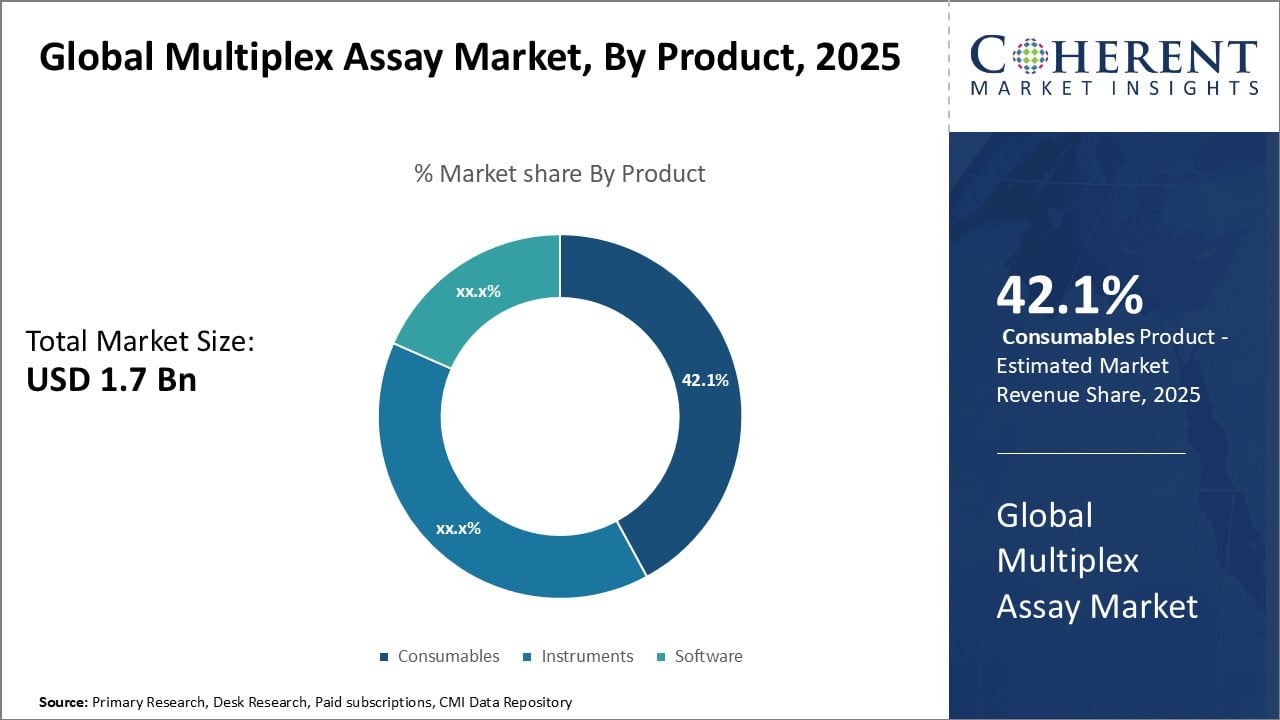
Figure 2. Global Multiplex Assay Market Share (%), By Product, 2025
To learn more about this report, Download Free Sample
Top Companies in the Multiplex Assay Market:
- Luminex Corporation
- Thermo Fisher Scientific, Inc.
- Illumina, Inc.
- Bio-Rad Laboratories, Inc.
- Qiagen
- Abcam Plc.
- Becton, Dickinson and Company
- Merck KGaA
- Agilent Technologies
- MESO SCALE DIAGNOSTICS, LLC.
- Randox Laboratories Ltd.
- Seegene Inc
- Perkin Elmer Inc.
- R&D Systems, Inc.
- Advanced Cell Diagnostics, Inc.
Definition:
A multiplex assay is a laboratory procedure that allows the simultaneous measurement and detection of multiple biological analytes (such as DNA, RNA, proteins, or enzymes) in a single sample. This technique is valuable because it increases throughput and conserves sample while providing comprehensive data about the interactions between numerous biological systems. Multiplex assays can be used in various fields including clinical diagnostics, research, and drug development.
Few Other Promising Reports in the Pharmaceutical Industry
Drug Discovery Outsourcing Market
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




