Global Inhalable Drugs Market is estimated to be valued at USD 40.06 Bn in 2025 and is expected to reach USD 65.17 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.2% from 2025 to 2032.
Inhalable drugs refer to medications that are administered via the respiratory tract for both local and systemic effects. These drugs are classified into two main types - aerosols and dry powder inhalers. Aerosol inhalers contain medications in solution or suspensions that are released as fine particle mist upon activation. Examples include albuterol and ipratropium bromide for respiratory diseases. They provide quick relief but require coordination to inhale correctly.
Dry powder inhalers, on the other hand, contain medication powders that are dispersed as fine particles upon inhalation. Popular categories under this include corticosteroids and long-acting beta agonists that are used to control asthma and chronic obstructive pulmonary disease (COPD) symptoms. Devices like Turbuhaler and Diskus do not require propellants and are breath-actuated, thus making them easier to use as compared to aerosols. However, their efficacy depends on inspiratory flow rates which may not be optimized in all patients.
Global Inhalable Drugs Market- Regional Insights
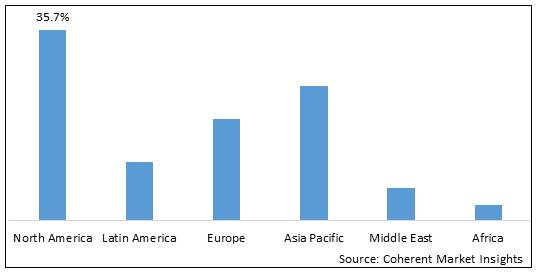
- North America is expected to be the largest market for inhalable drugs during the forecast period, accounting for over 35.7% of the market share in 2025. North America dominates the global inhalable drugs market due to strong industry presence and higher adoption rates. U.S. is home to majority of top pharmaceutical companies with established research and development (R&D) centers focusing on respiratory diseases. Several blockbuster drugs were first developed and launched in the U.S., gaining early market access and wide acceptance among physicians and patients. With a large healthcare expenditure and growing aging population prone to respiratory issues, the U.S. market provides lucrative business opportunities for innovator companies to recover investments made in drug development. However, drug prices in the U.S. are among the highest globally which also encourages generics market.
- Asia Pacific market is expected to be the second-largest market for inhalable drugs, accounting for over 25.2% of the market share in 2025. The Asia Pacific region is expected to be the fastest growing market for inhalable drugs worldwide. Countries like China, India, and Japan are primed for high growth on account of expanding middle class, increasing healthcare expenditure and rising incidence of diseases like asthma and COPD. Their huge combined population of over four billion people represents substantial market potential. Further boosting industry activity in the region is the growth of domestic pharmaceutical firms with expanded manufacturing capabilities as well as research focus on respiratory therapeutics. Several multinational companies have also set up manufacturing plants in Asia to cater to the regional demand and benefit from lower costs of production. This has made inhalable drugs more affordable and accessible to large patient segments. Trade relationships between Asia Pacific countries ensure steady imports and exports within the region by supplementing overall market volumes.
- Europe market is expected to be the fastest-growing market for inhalable drugs, with a share of 19% during the forecast period. The growth of the market in Europe is due to the increasing prevalence of asthma in the region.
Analyst View: Global inhalable drugs market has strong growth potential driven by the rising prevalence of respiratory diseases such as asthma and COPD. Inhaled drugs allow for direct delivery of medications to the lungs, thereby improving efficacy and reducing the risk of side effects that are associated with other administration routes. Technological advancements in drug delivery devices will further support market gains. Compact and portable inhalers offering targeted dose delivery will see greater patient acceptance.
Figure 1. Global Inhalable Drugs Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Inhalable Drugs Market- Drivers
- Growing prevalence of respiratory diseases: The key driver contributing to the growth of the global inhalable drugs market is the increasing prevalence of respiratory diseases across the world. Respiratory diseases have emerged as a major public health concern, thereby affecting people of all age groups. Some of the most common respiratory diseases include chronic obstructive pulmonary disease (COPD), asthma, lung cancer, pneumonia, and others. On the other hand, approximately 339 million people suffer from asthma every year globally. The prevalence of asthma has increased significantly over the past few decades, especially in developing nations.
- A number of factors have led to the growth in respiratory diseases. Rising levels of air pollution in cities and industrial areas have negatively impacted lung health. Long-term exposure to pollutants releases inflammatory particles and chemicals in the lungs thus causing inflammation and breathing difficulties over time. Growing aging population also contributes as elderly people are more vulnerable to respiratory illnesses. Changing lifestyle and dietary patterns have increased obesity rates which is a risk factor for asthma and COPD. With growing environmental degradation and toxic emissions in the atmosphere, the cases of respiratory diseases are expected to spike further.
- The rising disease burden has augmented the demand for drugs that can be effectively delivered to the lungs. Inhalable medication allows direct delivery of drugs to the primary disease site and helps achieve better therapeutic outcomes. Pulmonary route of drug administration leads to fewer systemic side effects as compared to oral drugs. This has prompted drug manufacturers to expand their inhalable drugs portfolio with advanced formulations for effective management of respiratory disorders.
- Advancement in delivery technologies: Constant innovation and advancement in drug delivery technologies have significantly contributed to the growth of the inhalable drugs market. The field of pulmonary drug delivery has evolved over the years with continuing research on new methodologies and formulations for achieving optimal drug deposition in the lungs. Novel technologies have enabled development of easy-to-use, portable inhalers along with sophisticated drug containment and release mechanisms for achieving improved safety, efficacy, and patient compliance.
- Traditional pressurized metered dose inhalers (pMDIs) are being replaced with more user-friendly dry powder inhalers (DPIs) which are breath-actuated and do not require coordination between actuation and inhalation. Further, smart inhalers integrated with sensors and connectivity features provide automated dose counting and tracking along with remote monitoring capabilities. This has benefits like ensuring adherence and timely refills. Injection molding technology is being used for manufacturing inhalable capsules and miniaturized formulations. Also, advanced pulmonary drug delivery systems utilizing micro and nanotechnologies can potentially deliver drugs for local as well as systemic treatments.
- Biopharmaceutical companies are continually introducing novel propellant-free inhalers, breath-controlled reservoirs, and other cutting-edge technologies tailored for specific drug molecules and disease conditions. Such innovations not only improve lung deposition but also enhance patient experience and compliance to drive the demand for inhalable drugs. With ongoing R&D focusing on next-gen technologies, the delivery systems are expected to become more efficient and customized in the future.
- Ease of drug delivery via inhalation: Global inhalable drugs market is witnessing significant growth due to increasing ease of drug delivery through the pulmonary route of administration. Inhalable drugs offer convenience of self-administration to patients and help avoid complications that are associated with other delivery methods like injections. This makes inhalation a preferred choice for treatment of various chronic diseases.
- Asthma and COPD are among the major disease areas driving the demand for inhalable drugs. in 2020, According to the report published by the World Health Organization (WHO), asthma affected over 339 Mn people worldwide. The prevalence of COPD is also on the rise and is projected to be the third leading cause of death globally by 2030. Inhaled medications have proved to be more effective in treating symptoms and controlling these respiratory diseases as compared to oral drugs. The effectiveness and ease of use that are associated with inhalable formulations is resulting in higher patient compliance for asthma and COPD treatments.
- The market is expected to experience increased growth led by innovations to develop more user-friendly and compact drug-device combination products. Companies are investing in connected digital inhalers that can record medication usage data to improve therapy management. This futuristic approach will enhance patient engagement and therapy outcomes. For example, the Tesseract predictive inhaler developed by GSK plc. Collects usage data to notify healthcare providers in case of incorrect inhalation and non-adherence. Moreover, many pipeline drugs for respiratory and non-respiratory diseases are being evaluated in inhalable formulations which will further augment market expansion, if approved.
Global Inhalable Drugs Market- Opportunities
- Emergence of new drug delivery technologies: Emergence of innovative drug delivery technologies can unlock tremendous opportunities in the global inhalable drugs market in the near future. Traditional delivery methods through pressurized metered dose inhalers and dry powder inhalers have certain limitations in terms of drug potency, precision of dosing, and patient adherence. However, newer technologies like soft mist inhalers and innovative capsule-based systems are helping to overcome many of these challenges.
- Soft mist inhalers utilize hydrofluoroalkane propellants which allow creation of a very fine mist of medication that deposit deeply into the lungs. This ensures maximum absorption of the active drug. Capsule-based devices ensure precise filling of the right dosage and also help avoid inconsistencies that can arise from loose powder formulation. The capsule shells dissolve instantaneously on contact with the moisture in lungs, thereby releasing the drugs for efficient absorption.
- Rising healthcare expenditure: The significant factor influencing the growth rate of the global inhalable drugs market is the growing healthcare expenditure, which helps in improving its infrastructure. For instance, according to the International Health Care System of the U.S., in June 2020, U.S. government organizations aim to improve the healthcare infrastructure by increasing funding, setting legislation, and national strategies, and cofounding and setting basic requirements and regulations for the Medicaid program. Similarly, in November 2022, according to reports provided by the Canadian Institute for Health Information, the total health expenditure in Canada was US$ 331 billion in 2022, or US$ 8,563 per Canadian, while health expenditure represented 12.2% of Canada's gross domestic product (GDP) in 2022, following a high of 13.8% in 2020.
- Potential for biologics delivery through inhalation: Potential for biologics delivery through inhalation in global inhalable drugs market brings an immense opportunity for growth in the near future. Biologics have revolutionized treatment across several therapeutic areas like oncology, immunology, and others. However, delivery of biologics still remains a challenge due to their large molecular structure and instability. Inhalation offers non-invasive and direct delivery of biologics to lungs which has potential to revolutionize treatment of respiratory diseases.
- Inhalation allows for deep lung deposition of biologics. Many respiratory diseases manifest in lungs, thereby making inhalation an attractive route for targeting drug delivery. For example, on May 16, 2023, according to the report published by the World Health Organization (WHO), , chronic obstructive pulmonary disease (COPD) affected over 3 Mn people and caused over 3 lakh deaths worldwide in 2019. Inhalation allows biologics to reach infection sites in lungs to treat underlying causes of diseases like COPD. This reduces systemic side effects and improves treatment efficacy with lower doses.
Global Inhalable Drugs Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 40.06 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.2% | 2032 Value Projection: | USD 65.17 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
GSK plc., Mundipharma International., Boehringer Ingelheim International GmbH., Cipla Inc., AstraZeneca, Sanofi, Vectura Group Ltd., Viatris Inc., 3M Health Care, Allied Healthcare Products, Inc., CareFusion Corporation, GF Health Products, Inc., Merck & Co., Inc., Novartis AG, Omron Healthcare, Inc., Glenmark Pharmaceuticals and PARI Respiratory Equipment, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Inhalable Drugs Market- Trends
- Adoption of smart inhalers: The use of smart inhalers is growing significantly worldwide as they provide better management of respiratory diseases like asthma and COPD. Smart inhalers contain digital sensors that can track medication use and send data to the patient's smartphone via an app. This allows doctors to remotely monitor patients and ensure that they are using their inhalers correctly and regularly as prescribed. It gives patients real-time feedback to prevent attacks and manage their condition better.
- The digitally connected nature of smart inhalers is revolutionizing how inhalable drugs are prescribed and consumed. Doctors can make timely interventions that are based on usage data to reduce exacerbations and hospitalizations for patients. Pharmaceutical companies are also able to gather valuable insights into medication adherence patterns. This enables them to engage better with patients through apps and customize treatment plans. Over time, widespread adoption of smart inhalers could see a transition towards more digital therapeutics that leverages comprehensive data on symptoms and lifestyle factors.
- Growing preference for digital health solutions: Global inhalable drugs market is witnessing a growing preference for digital health solutions which can provide significant opportunities for growth. With the COVID-19 pandemic accelerating the adoption of telehealth and remote patient monitoring solutions, more patients are comfortable with using digital platforms to manage chronic conditions like asthma and COPD. This trend is expected to continue even in the post-pandemic era as people have realized the advantages of convenience, low costs, and reduced risk of infections that are associated with digital healthcare
- Vendors in the inhalable drugs market are developing various digital solutions to engage with patients in a more effective way. They are integrating inhaler sensors and mobile apps that can track medication adherence, monitor symptoms remotely, and alert caregivers in case of emergencies. For example, Propeller Health's smart inhaler sensors paired with a digital platform allows doctors to download usage data and customize treatment plans in real-time for better management of respiratory diseases. Such solutions ensure patients in facing no disruption in care while maintaining social distancing and help providers proactively in addressing non-adherence issues.
Global Inhalable Drugs Market - Restraints
- Risk of misuse of inhalable medicines: Risk of misuse and abuse of inhalable medicines is a significant concern restraining the growth of the global inhalable drugs market. Inhalable drugs, if misused, can lead to adverse health issues and even life-threatening conditions in some cases. The misuse of these drugs involves inhaling them via unauthorized routes of administration such as sniffing or smoking rather than using an inhaler or nebulizer as prescribed. This often stems from the misunderstanding or ignorance regarding the appropriate usage of these medicines and an inability to precisely follow directions for use.
- The misuse of inhalable drugs can cause several problems. For instance, inhaling corticosteroids through improper routes like sniffing them can lead to conditions like throat irritation, nosebleeds, and nausea. Similarly, inhaling bronchodilators meant for oral inhalation through routes, such as sniffing, can result in irregular heartbeat, dizziness, and nervousness. In some instances, the misuse has also been associated with fatal outcomes. For example, according to the reports by the National Institute on Drug Abuse, emergency room visits involving the non-medical use of inhalants rose from 1.26 Mn in 2020 to 1.34 Mn in 2021, thus underscoring the severity of the problem.
- Counterbalance: The correct use of inhalable drugs needs to be made aware by the key market players among the people.
- High development costs: Developing an inhalable drug requires extensive research and testing over many years. This drug development process involves discovery, pre-clinical research, clinical research, regulatory review and approval, and product commercialization. Each phase of the drug development process demands significant financial investments running into hundreds of millions of dollars.
- For example, according to data by the U.S. Food and Drug Administration (FDA) in the U.S, the average out-of-pocket cost to develop a new drug was estimated to be US$ 2.64 billion in the year 2020. This cost takes into account the research and development expenditures across various stages from drug discovery to approval. A significant portion of this cost is use to clinical trials where drugs have to undergo rigorous testing on human subjects to establish safety and efficacy. Any failures or setbacks during the clinical trial phases results in tens or hundreds of millions of dollars in additional costs for drug developers.
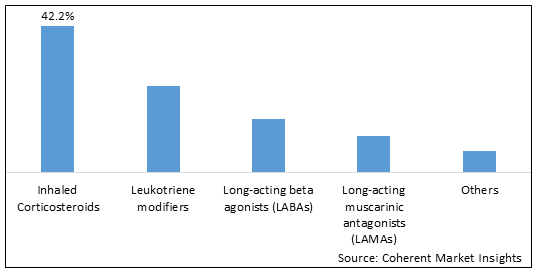
Figure 2. Global Inhalable Drugs Market Share (%), By Drug Class, 2025
To learn more about this report, Download Free Sample
Global Inhalable Drugs Market- Recent Developments
Product Approval and Launch
- On July 31, 2023 Viatris Inc., a global healthcare company, and Kindeva Drug Delivery L.P., a pharmaceutical contract development and manufacturing organization, launched Breyna (budesonide and formoterol fumarate dihydrate) inhalation aerosol, the first generic version of AstraZeneca's Symbicort with an abbreviated new drug application (ANDA) approved by the U.S. Food and Drug Administration (FDA). Breyna, a drug-device combination product, is indicated for certain patients with asthma or chronic obstructive pulmonary disease (COPD) and will be immediately available in both 80 mcg/4.5 mcg and 160 mcg/4.5 mcg dosage strengths.
- In September 2020, GlaxoSmithKline plc., a global healthcare company and Innoviva, Inc., a pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) had approved a new indication for Trelegy Ellipta (fluticasone furoate / umeclidinium / vilanterol ‘FF/UMEC/VI’) for the treatment of asthma in patients aged 18 years and older adding to its current license for use in patients with chronic obstructive pulmonary disease (COPD). Trelegy Ellipta is not indicated for relief of acute bronchospasm. The U.S. FDA-approved strength for both chronic obstructive pulmonary disease and asthma is fluticasone furoate / umeclidinium / vilanterol 100/62.5/25mcg. There is an additional strength for asthma alone which is fluticasone furoate / umeclidinium / vilanterol 200/62.5/25mcg.
Research and Development Activities by the Market Players
- On November 27, 2023, Sanofi, a pharmaceutical company, announced that Dupixent significantly reduced COPD exacerbations in second positive Phase 3 trial, accelerating U.S. Food and Drug Administration submission and confirming potential to become first approved biologic for this serious disease
- In November 2022, PT027 had been recommended by the U.S.FDA Advisory Committee as new rescue treatment for asthma. T027 is a potential first-in-class, pressurized metered-dose inhaler (pMDI), fixed-dose combination rescue medication in the U.S. containing albuterol, a short-acting beta2-agonist (SABA), and budesonide, an anti-inflammatory inhaled corticosteroid (ICS). It is being developed by AstraZeneca, a pharmaceutical company and Avillion, a global drug development company.
Top Companies in Global Inhalable Drugs Market
- GSK plc.
- Mundipharma International.
- Boehringer Ingelheim International GmbH.
- Cipla Inc.
- AstraZeneca
- Sanofi
- Vectura Group Ltd.
- Viatris Inc.
- 3M Health Care
- Allied Healthcare Products, Inc.
- CareFusion Corporation
- GF Health Products, Inc.
- Merck & Co., Inc.
- Novartis AG
- Omron Healthcare, Inc.
- Glenmark Pharmaceuticals
- PARI Respiratory Equipment, Inc.
Definition: Inhalable drugs refer to pharmaceutical products that can be administered through the respiratory tract via the lungs. There are primarily two main types of inhalable drugs - inhaled aerosols and nasal sprays. Inhaled aerosols are usually fine drug particles or liquid droplets that can be delivered deep into the lungs through devices such as metered-dose inhalers (MDIs) and dry powder inhalers (DPIs). Common inhaled aerosol drugs treat conditions like asthma and COPD. They work fast to deliver medicine directly to the airways for quick symptom relief. However, improper inhalation technique can compromise drug delivery to the lungs. Nasal sprays, on the other hand, carry medication in an aqueous or oily solution which is administered intranasally for local or systemic effects. Allergy nasal sprays are one such example.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




