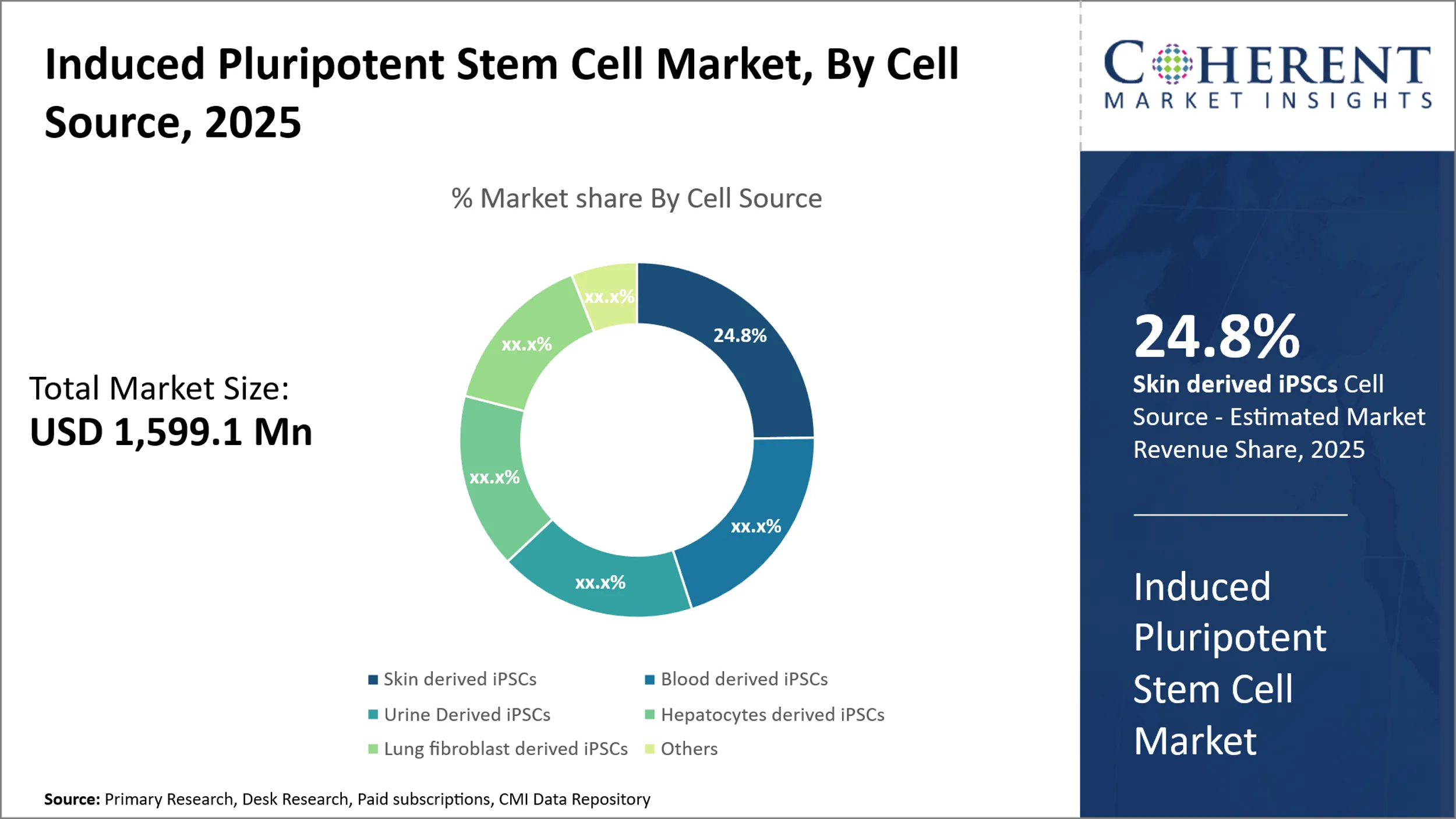
Global Induced Pluripotent Stem Cells Market Size and Share Analysis- 2025-2032
The global induced pluripotent stem cells market was valued at USD 1,599.1 Mn in 2025 and is expected to reach USD 3,383.1 Mn by 2032, growing at a compound annual growth rate (CAGR) of 11.3% from 2025 to 2032.
Key Takeaways
- Based on Cell Sources, Skin-Derived IPSCs Segment dominate the global market with a 24.8% share. This is due to their accessibility, minimal invasiveness, and high reprogramming efficiency.
- North America is expected to be the largest market for induced pluripotent stem cells over the forecast period and it accounted for over 36.3% of the market share in 2025. This is due to the presence of leading medical device companies, highly established healthcare infrastructure, and significant healthcare expenditure in the region.
- Europe will be the second-largest market for induced pluripotent stem cells, which accounted for over 30.4% of the market in 2025. This follows the fact that several research studies at universities and hospitals in most major European countries like Germany, U.K., and France.
Market Overview
Induced pluripotent stem cells (iPSCs) industry is propelled by innovation in regenerative medicine, tailored therapies, and disease modeling. iPSCs enable the transformation of adult cells to a pluripotent condition, thereby allowing for the generation of patient-specific cell therapies and avoiding the ethical issues surrounding embryonic stem cells. Application in drug development, toxicity testing, and tissue engineering is increasingly desired, especially in the framework of neurological, cardiovascular, and metabolic diseases. Increased investment in stem cell research, along with favorable regulatory environments within the North American and European markets, is also driving market growth.
Current Events and Its Impact on Induced Pluripotent Stem Cells Market
|
Current Events |
Description and its impact |
|
StemNext Therapeutics Launches First AI-Directed iPSC-Based Therapy for Parkinson's Disease |
|
|
EU Approves Public-Private Consortium for iPSC Biobanking and Drug Screening
|
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Induced Pluripotent Stem Cells Market Insights, By Cell Sources
Based on cell sources, Skin-derived iPSCs dominate the global market with a 24.8% share due to their accessibility, minimal invasiveness, and high reprogramming efficiency. Skin fibroblasts are easy to obtain through biopsies, making them ideal for patient-specific iPSC generation. They have well-established protocols and lower ethical concerns. Their stability and responsiveness to reprogramming methods enhance consistency. As a result, they are widely used in disease modeling, drug screening, and regenerative therapies.
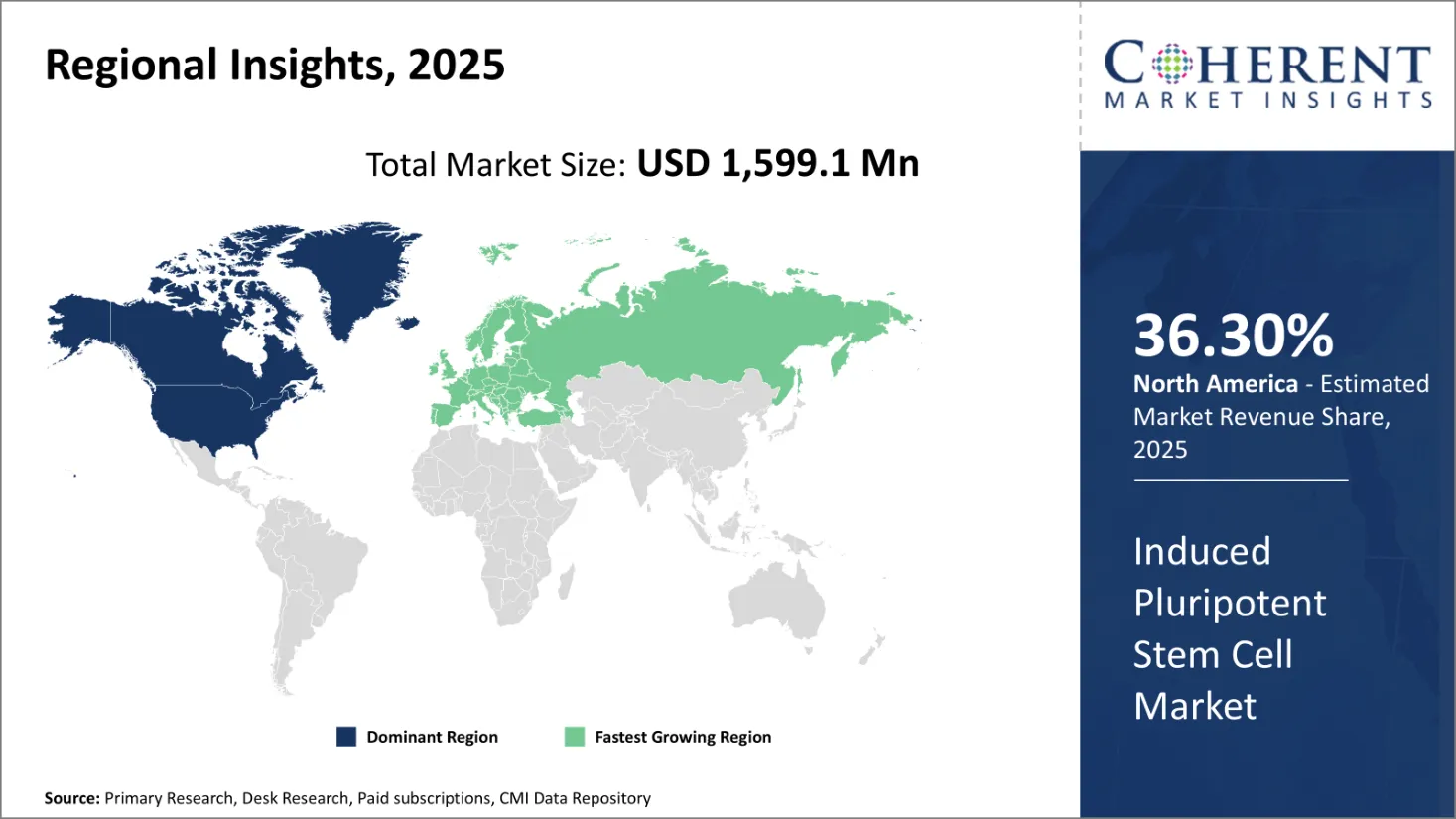
Regional Insights
To learn more about this report, Download Free Sample
Global Induced Pluripotent Stem Cells Market Regional Insights
North America Induced Pluripotent Stem Cells Market Analysis & Trends
North America is expected to be the largest market for induced pluripotent stem cells over the forecast period and it accounted for over 36.30% of the market share in 2025. The growth of the market in North America is due to the presence of leading medical device companies, established healthcare infrastructure, and high healthcare expenditure, have driven market growth over the years.
Europe Induced Pluripotent Stem Cells Market Analysis & Trends
Europe is expected to be the second-largest market for induced pluripotent stem cells, which accounted for over 30.4% of the market share in 2025. The growth of the market is due to the presence of several ongoing research initiatives at universities and hospitals across major European countries like Germany, U.K., and France have established standardized protocols for efficiently reprogramming blood cells into iPSCs.
Global Induced Pluripotent Stem Cells Market Outlook Country-Wise
United States Induced Pluripotent Stem Cells Market Analysis and Trend
The United States leads the induced pluripotent stem cells (iPSCs) market due to its robust biotechnology industry, substantial research investments, and participation of leading pharmaceutical companies and academic institutions. Government support and encouraging regulatory environments foster innovation in regenerative medicine and cell therapy research.
Japan Induced Pluripotent Stem Cells Market Analysis and Trend
Japan is a global leader in the market of iPSC and was previously recognized for stem cell pioneering research. The country has beneficial policies to promote clinical applications of iPSC-based therapies, especially for disease related to aging, and serves as an anchor for translational research and business development.
China Induced Pluripotent Stem Cells Market Analysis and Trend
China is rapidly building its iPSC market with significant investment in biotechnology facilities and stem cell research programs. Its growing network of research centers and collaborations with foreign firms is driving progress in disease modeling and regenerative medicine.
Germany Induced Pluripotent Stem Cells Market Analysis and Trend
Germany stands out in Europe for its strong focus on stem cell research and regenerative medicine. Strong pharmaceutical and healthcare industries, coupled with government support, form the basis of significant advancements in iPSC research for pharmaceutical and clinical applications.
South Korea Induced Pluripotent Stem Cells Market Analysis and Trend
South Korea has significantly developed iPSC technology, thanks to governmental backing and collaborative partnerships between academicians and industry players. Its emphasis on regenerative medicine and personalized therapies situates it as a key market player in Asia.
Market Report Scope
Induced Pluripotent Stem Cells Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,599.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.3% | 2032 Value Projection: | USD 3,383.1 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Takara Bio Inc., Thermo Fisher Scientific, Fujifilm Holdings Corporation, Astellas Pharma, Fate Therapeutics, Ncardia, ViaCyte, Cellular Dynamics International, Lonza, Blueprint Medicines and Other Prominent Players |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Induced Pluripotent Stem Cells Market Drivers
- Increasing research funding
Government and private funding for stem cell research has increased substantially in recent years, fueling innovations with induced pluripotent stem cells (iPSCs) that are expanding their applications and driving more research. Countries like Japan, China, and several nations in Europe have significantly boosted budgets for stem cell science, with a particular emphasis on iPSC technology's potential role in developing personalized regenerative medicines.
- Growing prevalence of neurodegenerative diseases
Induced pluripotent stem cell technology has emerged as a promising avenue for developing novel treatments targeting the underlying causes of these incurable diseases. iPSCs offer a potential alternative to human embryonic stem cells for achieving personalized cell therapies and developing neurodegenerative diseases models for drug discovery efforts.
- Surging demand for personalized medicine
Many pharmaceutical companies are investing heavily in iPSC research to develop biomarkers for disease diagnosis and monitoring treatment response. They are using iPSC-derived cells from disease-specific donors to validate drug targets and toxicity. For example, Astellas Pharma, a Japan based multinational pharmaceutical company, established disease modeling and drug discovery platforms by using iPSCs from amyotrophic lateral sclerosis and Duchenne muscular dystrophy patients.
Several clinical trials are ongoing to evaluate the safety and efficacy of iPSC-derived cells for retinal dystrophy, spinal cord injury, heart disease, and cartilage damage. The success of these studies will demonstrate the potential of iPSCs to transform regenerative medicine from a research concept to an established clinical solution.
- Rising numbers of clinical trials
More research institutes and pharmaceutical companies are conducting clinical trials to evaluate the therapeutic potential of iPSCs. For instance, according to the World Health Organization (WHO), over 300 iPSC clinical trials were registered between 2020-2022 worldwide targeting a variety of conditions like macular degeneration, Parkinson's disease, heart disease and diabetes.
Countries with most registered iPSC clinical trials during this period included Japan, USA, China, and U.K. These trials are investigating the efficacy and safety of using iPSC-derived cells for transplantation, drug testing, and disease modeling. Successful trial outcomes could validate their use in the clinic in the near future.
Global Induced Pluripotent Stem Cells Market Opportunities
- Increasing collaborations and partnerships between research institutes and pharma companies
The field of regenerative medicine has experienced significant advancements in recent years due to growing stem cell research. More research institutes and pharmaceutical companies are conducting clinical trials to evaluate the therapeutic potential of iPSCs.
Countries with most registered iPSC clinical trials during this period included Japan, U.S., China and U.K. These trials are investigating the efficacy and safety of using iPSC-derived cells for transplantation, drug testing, and disease modeling. Successful trial outcomes could validate their use in the clinic in the near future.
- Growing application areas in regenerative medicine
Regenerative medicine has the potential to revolutionize treatment for various degenerative diseases and injuries. The ability of induced pluripotent stem cells (iPSCs) to differentiate into any cell type in the body makes them incredibly useful for regenerative therapies. This could be a major growth opportunity for the global iPSC market.
Some key therapeutic areas that are actively researching iPSC applications include musculoskeletal disorders, neurodegenerative diseases, cardiovascular diseases, diabetes, and ocular diseases. For example, iPSC-derived retinal epithelial cells are being tested in clinical trials to treat age-related macular degeneration, one of the leading causes of blindness worldwide. Ipsaga, a biotech company, has also had success by using iPSC-derived chondrocytes to regenerate cartilage in animal models of osteoarthritis.
As the science progresses, additional clinical applications are sure to emerge for regenerative treatments of cartilage, bone, heart, pancreatic islets, and various neurological tissues.
- Scope for development of advanced therapies using iPSCs
The capabilities of iPSCs to turn into any cell type of the body have attracted significant investments from pharmaceutical and biotechnology companies. They are working towards developing iPSC-based therapies and products across different therapeutic categories like neurodegenerative disorders, blood disorders, and diabetes.
Successful clinical trials and approvals will demonstrate the real-world applicability of this technology. Commercialization of first few iPSC products will prove to be a landmark achievement and significantly boost the future research pipelines.
Government agencies like the National Institutes of Health, U.S. and National Institute for Health Research, U.K. are supporting large number of iPSC projects through funding.
Global Induced Pluripotent Stem Cells Market Trends
- Increasing focus on development of Xeno-free culture media
Many researchers and industry players are investing resources to engineer Xeno-free solutions for stem cell maintenance and differentiation. Several bio-tech startups as well as large corporations like Thermo Fisher Scientific, a biotechnology and laboratory equipment company introduced human extracellular matrix-based culture plates that facilitate the growth of iPSCs in nutrient solutions devoid of animal feeders, serum and other xenogeneic materials.
- Rising adoption of 3D cell culture technology
Rising adoption of 3D cell culture technology is having a significant impact on the global induced pluripotent stem cells (iPSCs) market. 3D cell culture allows for the growth of living cells in all three dimensions, which better mimics the natural cellular environment in the human body as compared to traditional two-dimensional cell cultures. This opens up new possibilities for disease modeling and drug development by using iPSCs.
Many researchers and pharmaceutical companies are utilizing 3D cell culture techniques to develop more physiologically relevant in vitro disease and toxicity models by using iPSC-derived cells. This is helping yield results that translate better to in vivo human conditions.
- Growing preference for customer specific iPSC lines
Rising demand is encouraging more players to invest in developing advanced capabilities for generating large numbers of gene-edited and disease-relevant iPSC lines on a customer-specific basis. This is enabling outsourcing of certain research activities to specialized firms, thus reducing time and costs for academic institutes and pharmaceutical developers.
Recent Developments
- In November 2024, Sumitomo Pharma received FDA Investigational New Drug (IND) clearance to initiate Phase I/II clinical trials in the U.S. for DSP-3077, an allogeneic iPSC-derived retinal sheet therapy aimed at treating retinitis pigmentosa—marking a major step in iPSC-based ophthalmic treatments.
- In October 2024, Aspen Neuroscience expanded its manufacturing capabilities by opening a new 22,000 sq. ft GMP-grade facility in San Diego. The facility is dedicated to producing ANPD001, its iPSC-derived therapy for Parkinson’s disease, supporting its advancement toward clinical and commercial readiness.
Analyst Opinion ( Expert Opinion)
- Global induced pluripotent stem cells market is expected to grow significantly over the next decade driven by increasing stem cell research activities and investments. The ability of iPSCs to generate patient-specific cells provides opportunities for developing safer and more effective regenerative medicines. Furthermore, iPSCs can be used as a valuable research tool for drug discovery and toxicity screening applications.
- However, high costs that are associated with iPSC development and commercialization remain a major challenge to widespread adoption. Standardization of differentiation protocols is another bottleneck that needs to be addressed. Limited understanding of epigenetic changes during reprogramming is also a concern from efficacy and safety point of view. Market players should focus on refining techniques to generate clinical-grade iPSCs easily and cost-effectively.
- Industry-academia collaborations would help enhance applications of iPSC platforms. While regulations differ across regions, favorable regulations and guidelines would provide an impetus to the market. Ongoing stem-cell banking initiatives could help facilitate accessibilities to iPSC lines for research purposes.
Market Segmentation
- By Cell Source
- Skin derived iPSCs
- Blood derived iPSCs
- Urine Derived iPSCs
- Hepatocytes derived iPSCs
- Lung fibroblast derived iPSCs
- Others (Endothelial cells, Glial Cells, Adipose stem cells etc.)
- By Application
- Culture & Maintenance
- Differentiation
- Genetic Manipulation & Engineering
- Characterization & Validation
- Banking & Storage
- Others (Reprogramming kits, Analysis software etc.)
- By End User
- Hospitals
- Diagnostics Centers
- Pharmaceutical & Biotechnology Companies
- Contract Research Organizations
- Others (Academic & Research Institutes etc.)
- By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Top Companies in the Global Induced Pluripotent Stem Cells Market
- Takara Bio Inc.
- Thermo Fisher Scientific
- Fujifilm Holdings Corporation
- Astellas Pharma
- Fate Therapeutics
- Ncardia
- ViaCyte
- Cellular Dynamics International
- Lonza
- Blueprint Medicines
- Other Prominent Players
Sources
Primary Research Interviews
- Stem Cell Research Scientists and Principal Investigators
- Biotechnology and Pharmaceutical Company Executives
- Clinical Research Organizations (CROs) Representatives
- Regulatory Affairs Specialists
- Others
Databases
- ClinicalTrials.gov
- Global Health Observatory (GHO) Data Repository
- BioPharma Dive Intelligence Database
- Others
Magazines
- Nature Biotechnology Magazine
- Cell Stem Cell Magazine
- Genetic Engineering & Biotechnology News (GEN)
- BioWorld Intelligence Magazine
- Others
Journals
- Cell Stem Cell Journal
- Stem Cells and Development Journal
- Nature Methods Journal
- Others
Newspapers
- Financial Times
- The Wall Street Journal
- Reuters Health News
- Bloomberg Life Sciences
- Others
Associations
- International Society for Stem Cell Research (ISSCR)
- California Institute for Regenerative Medicine (CIRM)
- Alliance for Regenerative Medicine (ARM)
- European Medicines Agency (EMA)
- Others
Public Domain Sources
- U.S. Food and Drug Administration (FDA) Guidelines
- National Institutes of Health (NIH) Reports
- World Health Organization (WHO) Publications
- European Medicines Agency (EMA) Public Documents
- Others
Proprietary Elements
- CMI Data Analytics Tool
- Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients