Global Geriatric Medicines Market Size and Trends
Global geriatric medicines market is estimated to be valued at USD 1,073.13 Bn in 2025 and is expected to reach USD 1,701.91 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.8% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The geriatric population, which is the primary consumer of medicines meant for age-related diseases, is growing rapidly worldwide. Increased geriatric population base suffering from multiple co-morbidities is the major factor boosting demand for geriatric medicines. Furthermore, growing focus of pharmaceutical companies on developing specialized prescriptions for elderly care is also supporting the growth of the geriatric medicines market. However, the presence of generic drugs may hinder the market growth during the forecast period.
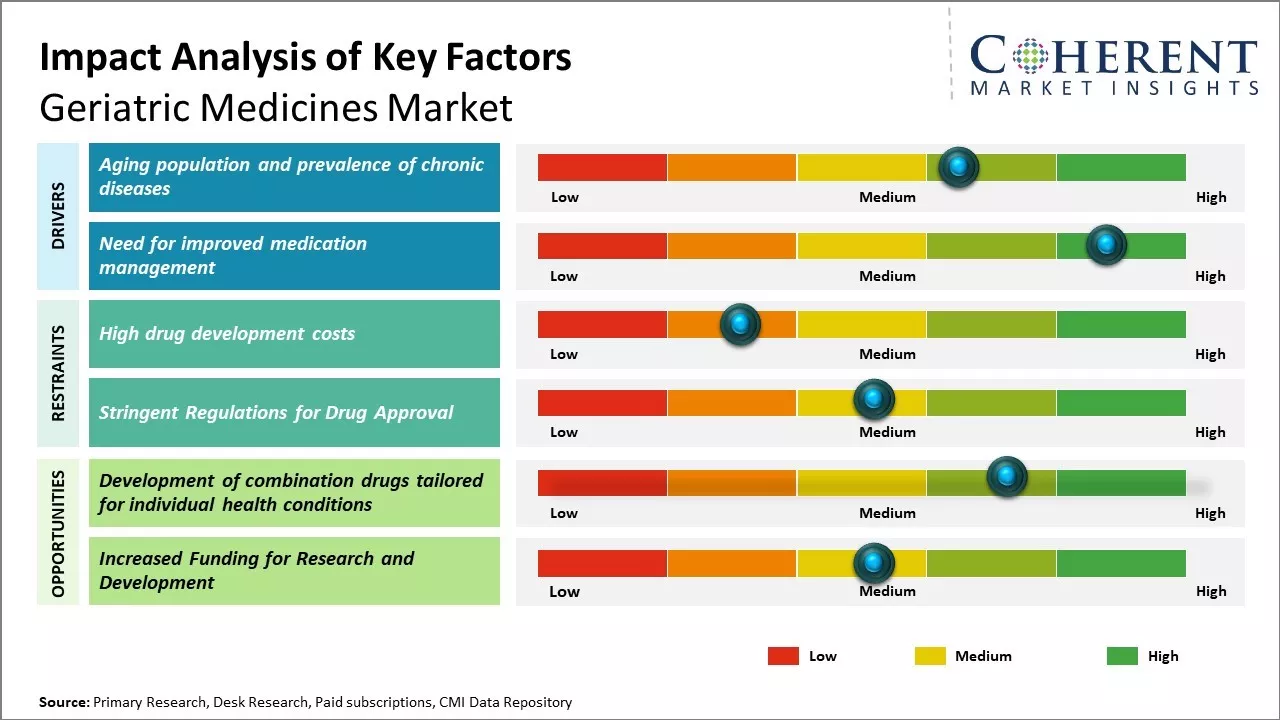
Aging population and prevalence of chronic diseases
As the average human lifespan continues to increase around the world, populations are witnessing an unprecedented growth in the number of elderly people aged 65 years and above. Aging leads to increased susceptibility to various chronic health conditions that often require long-term or lifelong management. Diseases such as cardiovascular disorders, cancer, diabetes, arthritis, Alzheimer's, Parkinson's, and others are highly prevalent among the geriatric demographic. The physiological changes associated with advancing age like reduced organ function, declined immunity and ability to heal present an added challenge in treatment. Furthermore, risk of polypharmacy also rises as elderly patients tend to suffer from multiple comorbidities concurrently. This growing prevalence of chronic illnesses boosts the need for a specialized range of pharmaceutical products that are tailored to the unique medical requirements of the aged population and can help improve their quality of life. Development of such geriatric-specific medicines aimed at effective management of age-related health issues can open lucrative opportunities for drug makers in the near future.
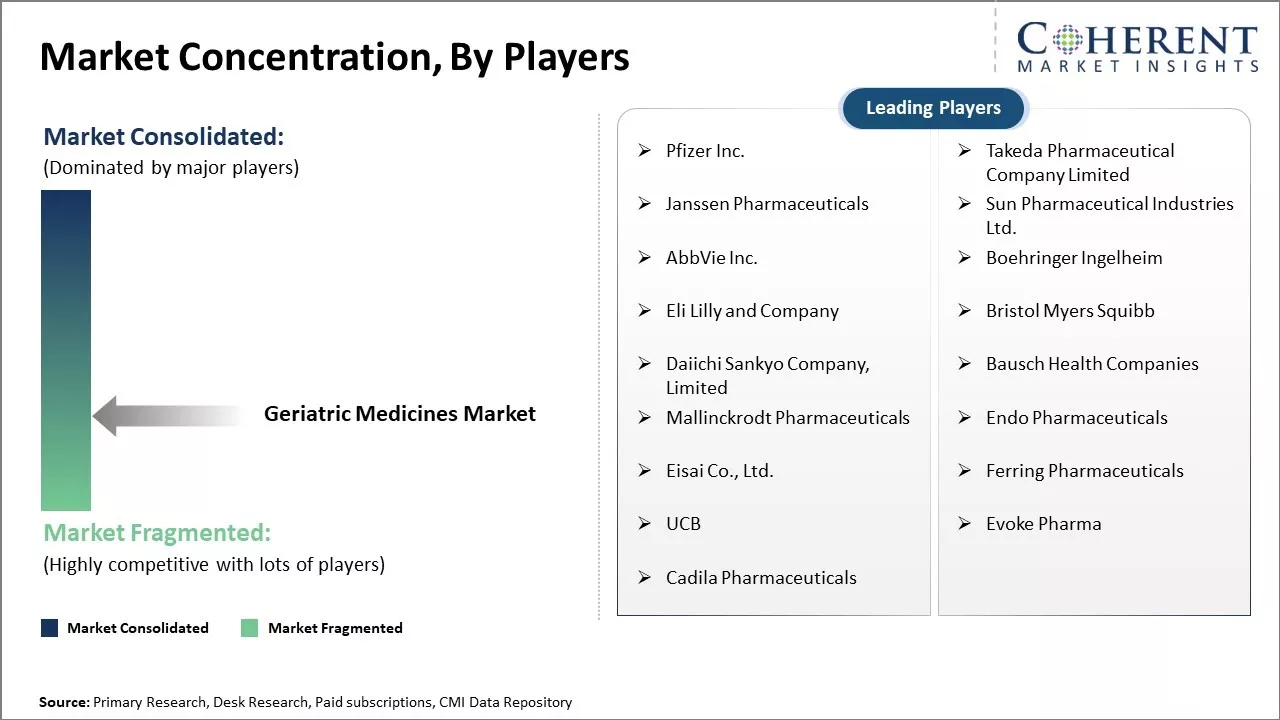
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Need for improved medication managementAnother critical driver for the geriatric medicines market is need for improved medication management among elderly individuals. Due to factors like reduced cognitive abilities, reduced organ function and higher vulnerabilities, medication regimens for senior citizens need to be designed very carefully. Issues pertaining to medication adherence, risk of adverse drug reactions and drug-drug interactions are much more common among geriatric patients on polypharmacy schedules. Factors like vision or hearing impairments can also negatively impact their ability to follow prescriptions properly, leading to treatment failures or health complications. There is growing emphasis on developing safer, easier to administer and more effective therapeutic solutions for this patient segment. Pharmaceutical companies are increasingly focusing on new delivery mechanisms, single pill combinations, customized dosing frequencies and diagnostics to facilitate optimized treatment support for the elderly population. This helps address an important clinical challenge and presents an opportunity for enhanced medical management of older demographics through innovation in geriatric drug formulations and solutions.

To learn more about this report, Download Free Sample
Market Challenges – High drug development costsGlobal geriatric medicines market faces several challenges. As the elderly population grows globally, age-related diseases are increasing which requires extensive research and development to find new treatment options. However, drug development costs are very high and bringing a new drug to the market is difficult and risky. Older patients often have multiple health issues, so testing treatments and managing side effects becomes more complex. Regulatory guidelines for clinical trials in elderly patients are stringent to ensure safety.
Market Opportunities – Development of combination drugs tailored for individual health conditions
The growth of the elderly population worldwide presents opportunities. As life expectancies increase, the demand for medicines to treat age-related chronic diseases will rise significantly. Manufacturers are working on developing safer drugs that can be used by those with co-morbidities. There is also scope for combinations of different drugs tailored for individual health conditions. Companion diagnostics can also help improve treatment outcomes for older patients, thereby, creating lucrative opportunities for market development over the projected period.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
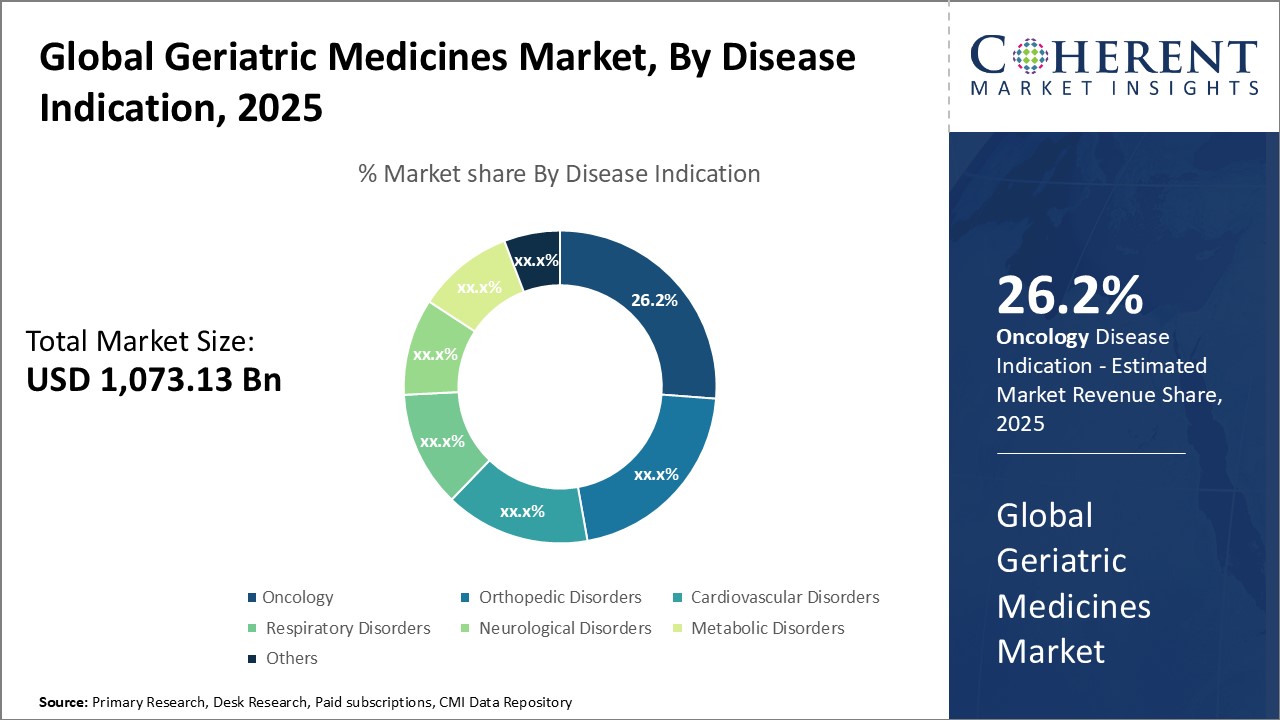
Insights, By Disease Indication: Aging population and high cancer incidence drives oncology segment growthDisease Indication segment is sub-segmented into oncology, orthopedic disorders, cardiovascular disorders, respiratory disorders, neurological disorders, metabolic disorders, and others. Oncology segment is estimated to hold 26.2% share of the market in 2025. As people grow older, their immune system weakens, making them more susceptible to developing cancer. Some of the common cancers affecting the elderly include breast cancer, prostate cancer, lung cancer, and colon cancer. With rising life expectancy around the world, the number of aging individuals is projected to surge dramatically over the coming decades. This burgeoning geriatric demographic will boost demand for oncology drugs designed specifically for older cancer patients. Moreover, certain types of cancers are predominantly associated with aging such as multiple myeloma which prompts heavy reliance on geriatric oncology medications. Stringent regulatory frameworks ensure the efficacy and safety of geriatric cancer treatments, further promoting their usage.
Insights, By Form: Prevalence of chronic diseases boosts tablet segment growth
Form segment is sub-segmented into tablet, liquid, capsule, inhalers, topical, and others. Tablet segment is anticipated to hold 38.3% of the market share in 2025. Older adults are more prone to develop long-term conditions like diabetes, heart disease, arthritis due to age-related physiological changes and immune system weaknesses. Tablets offer reliable and consistent dosage along with easy portability, swallowability and storage advantages, making them the preferred delivery format for managing chronic diseases over the long-term. Their solid unitary dosage form also facilitates adherence to complex drug regimens. Moreover, tablets production involves lower costs as compared to other dosage forms like liquid, thus, providing better price margins for pharmaceutical companies to cater to the massive target market of geriatric patients with diabetes, hypertension, and other chronic illnesses.
Insights, By Route of Administration: Ease of Administration Fuel Growth of Oral Administration Route
Route of Administration segment includes is sub-segmented into oral, parenteral, and others. Oral segment is anticipated to hold 57.2% of the market share in 2025. Due to aging, people develop certain medical conditions affecting swallowing ability such as dysphagia, arthritis or Parkinson's disease. Even minor swallowing difficulties can cause serious issues like choking, aspiration or malnutrition in the elderly. This prompts higher reliance on orally administered medicines which does not need specialized delivery methods. Moreover, oral drug formulations are easier to administer, have better acceptance and ensure higher compliance. With rising prevalence of dysphagia prevalence, global geriatric medicines market is advancing user-friendly or dispersible and chewable drugs to further boost convenience of oral route of administration.
Regional Insights
Need a Different Region or Segment? Download Free Sample
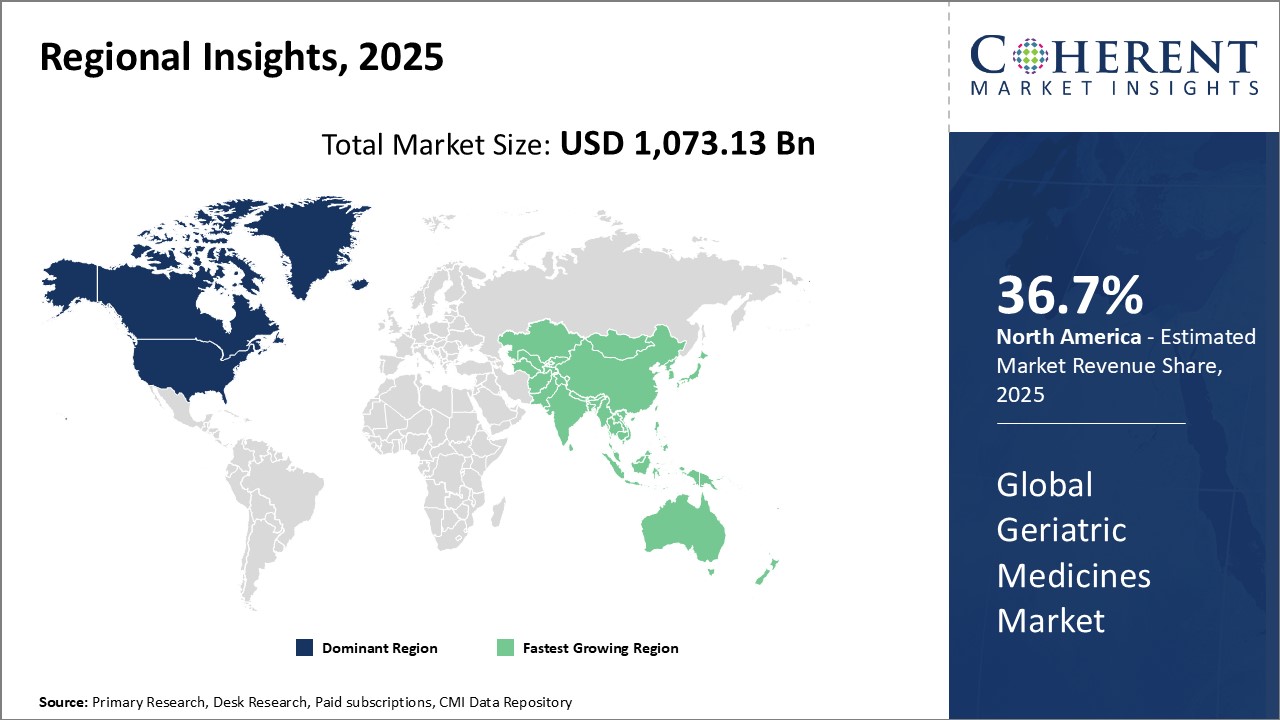
North America has established itself as the dominant region in the global geriatric medicines market and is anticipated to hold 36.7% of the market share in 2025. The high per capita healthcare expenditure in countries like U.S. and demand for innovative drugs to treat age-related chronic conditions have propelled the regional market growth. The U.S. alone accounts for over 65.5% of the North American market due to well-developed pharmaceutical industry and presence of major international drug makers in the region. In recent years, increasing preference for generic drugs to control escalating healthcare costs has emerged as a key trend in the regional market.
The Asia Pacific region is considered the fastest growing market for geriatric medicines driven by rapidly growing geriatric population, improving access to healthcare facilities and rising healthcare expenditure in emerging economies. Countries like China and India provide immense growth opportunities, owing to their huge geriatric demographic base and rising focus on quality healthcare by local governments. Several international drug manufacturers are shifting their manufacturing facilities to Asia in order to cater to regional demand in a cost-effective manner and benefit from policy incentives offered by governments. This has made APAC an important hub for generic drugs production. However, lack of comprehensive public insurance coverage in many South-East Asian countries remains a challenge for market expansion.
Market Report Scope
Global Geriatric Medicines Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1,073.13 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.8% | 2032 Value Projection: | USD 1,701.91 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., Takeda Pharmaceutical Company Limited, Janssen Pharmaceuticals, Sun Pharmaceutical Industries Ltd., AbbVie Inc., Boehringer Ingelheim, Eli Lilly and Company, Bristol Myers Squibb, Daiichi Sankyo Company, Limited, Bausch Health Companies, Mallinckrodt Pharmaceuticals, Endo Pharmaceuticals, Eisai Co., Ltd., Ferring Pharmaceuticals, UCB, Evoke Pharma, Cadila Pharmaceuticals |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Geriatric Medicines Industry News
- In May 2023, Pfizer Inc. announced that the U.S. Food and Drug Administration (FDA) has approved ABRYSVO (Respiratory Syncytial Virus Vaccine), the company's bivalent RSV prefusion F (RSVpreF) vaccine, for the prevention of lower respiratory tract disease caused by RSV in people aged 60 and older. ABRYSVO is an unadjuvanted vaccine made up of two preF proteins chosen to optimize protection against RSV A and B strains, and it has been shown to be both safe and efficacious.
- In May 2023, Otsuka Pharmaceutical, Co. Ltd., a pharmaceutical company and Lundbeck LLC, a pharmaceutical company, announced that the U.S. FDA had approved the supplemental New Drug Application (sNDA) of REXULTI (brexpiprazole) for use in the treatment of agitation associated with dementia due to Alzheimer’s disease
- In March 2023, The Penn Medicine Princeton Cancer Center received a USD 2.5 million grant from the Bristol Myers Squibb Foundation to fund an innovative program to ensure holistic, patient-centered care for older adults with cancer. The new Geriatric Oncology Program will transform cancer treatment and supportive care for older adults by expanding research opportunities, enhancing professionals’ expertise in geriatrics, and increasing outreach to seniors in the central New Jersey community.
- In January 2023, Eisai Co., Ltd., a pharmaceutical company, and Biogen Inc., a multinational biotechnology company, announced that the U.S. FDA has approved lecanemab-irmb 100 mg/mL injection for intravenous use, a humanized immunoglobulin gamma 1 (IgG1) monoclonal antibody directed against aggregated soluble ("protofibril") and insoluble forms of amyloid beta (Aβ) for the treatment of Alzheimer's disease (AD).
*Definition: Geriatric Medicines Market refers to the market for pharmaceutical drugs that are specifically designed, developed and tested for use in the elderly patient population aged 65 years and above. These medicines aim to prevent, treat or manage aging-related chronic conditions like cardiovascular diseases, cancer, diabetes, arthritis, neurological disorders and dementia. These also aim to improve quality of life by reducing the effects of aging on physiological functioning. Geriatric Medicines Market plays a crucial role in helping senior citizens live independently and healthy lives.
Market Segmentation
- Disease Indication Insights (Revenue, USD Bn, 2020 - 2032)
- Oncology
- Orthopedic Disorders
- Cardiovascular Disorders
- Respiratory Disorders
- Neurological Disorders
- Metabolic Disorders
- Others
- Form Insights (Revenue, USD Bn, 2020 - 2032)
- Tablet
- Liquid
- Capsule
- Inhalers
- Topical
- Others
- Route of Administration Insights (Revenue, USD Bn, 2020 - 2032)
- Oral
- Parenteral
- Intravenous
- Intramuscular
- Subcutaneous
- Others
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Pfizer Inc.
- Takeda Pharmaceutical Company Limited
- Janssen Pharmaceuticals
- Sun Pharmaceutical Industries Ltd.
- AbbVie Inc.
- Boehringer Ingelheim
- Eli Lilly and Company
- Bristol Myers Squibb
- Daiichi Sankyo Company, Limited
- Bausch Health Companies
- Mallinckrodt Pharmaceuticals
- Endo Pharmaceuticals
- Eisai Co., Ltd.
- Ferring Pharmaceuticals
- UCB
- Evoke Pharma
- Cadila Pharmaceuticals
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
