Global epinephrine autoinjector market is expected to reach US$ 4.95 Bn by 2032, from US$ 2.74 Bn in 2025, exhibiting a CAGR of 8.8% during the forecast period.
Epinephrine autoinjectors, commonly known as epinephrine pens or epipens, are handheld devices that contain a single dose of epinephrine, also known as adrenaline, and are used to treat severe allergic reactions also known as anaphylaxis. There are currently two main types of epinephrine autoinjectors available - single-dose prefilled syringes as well as single dose needle-free injectors.
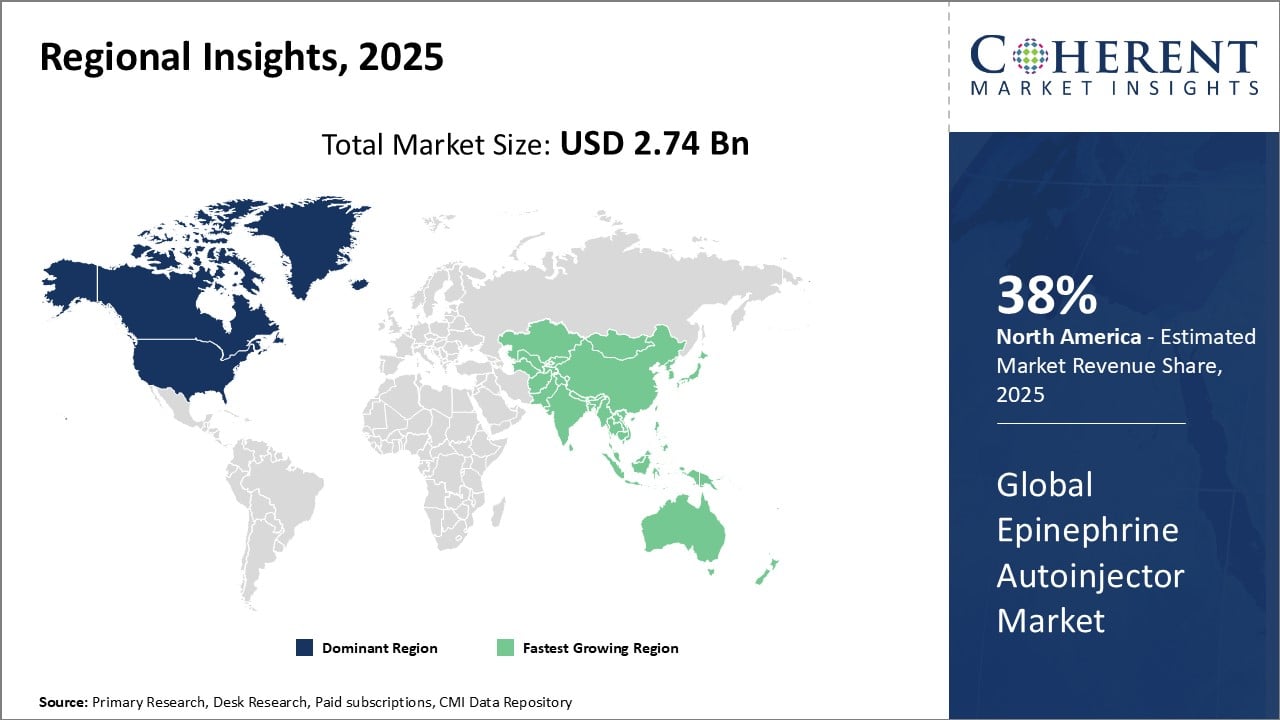
Global Epinephrine Autoinjector Market- Regional Insights
- North America is expected to be the largest market for epinephrine autoinjector during the forecast period, accounting for over 38.0% of the market share in 2025. North America has overwhelmingly dominated the global epinephrine autoinjector market for many years. The presence of major players like Mylan, Pfizer and several other pharmaceutical companies has established the region as the industry leader. North American countries accounted for over 45% share of the worldwide autoinjector device sales historically. Moreover, North America is the earliest adopter of innovative technologies and devices meant for emergency treatment of allergic reactions like anaphylaxis. This has ensured continuous supply and widespread adoption of autoinjectors within the region.
- Asia Pacific is expected to be the second-largest market for epinephrine autoinjector, accounting for over 24.4% of the market share in 2025. Countries like China, India and Japan are witnessing rising acceptance of epinephrine autoinjectors due to increasing awareness around life-threatening food and drug allergies. Asia Pacific has become an attractive investment destination for device manufacturers as the prevalence of allergies rises. Favorable regulations and increasing healthcare expenditure have encouraged both global companies and local players to expand their operations in the region. For instance, several North American companies have partnered with local pharmaceutical firms for easier market entry and distribution. This allows them to cater to the huge population base while benefiting from comparatively low production costs.
- Europe has promising market share of 17.0% during the forecast period. Epinephrine autoinjector market in Europe is witnessing significant growth due to rising incidences of severe allergic reactions and anaphylaxis. Increased awareness about the importance of prompt intervention and the convenience offered by autoinjectors contribute to market expansion. Key players in the region are focusing on technological advancements and strategic collaborations to enhance product offerings. Government initiatives promoting accessibility to epinephrine autoinjectors further boost market dynamics. With a growing emphasis on patient safety and emergency preparedness, the Europe epinephrine autoinjector market is poised for continued expansion, catering to the critical needs of individuals at risk of severe allergic reactions.
Analyst View:
Global epinephrine autoinjector market is poised to witness growth at a steady rate over the forecast period due to rising prevalence of food and other allergies globally. Growing consumer preference for self-administration devices to treat severe allergic reactions will boost sales of epinephrine autoinjectors. Furthermore, increasing patient awareness about anaphylaxis and availability of generic versions of branded autoinjectors are expected to propel the market growth. However, recurring product recalls by manufacturers owing to technical defects could hamper the market growth.
Regionally, North America will continue to dominate the market supported by strong demand for epinephrine autoinjectors in the U.S. The growing burden of allergies along with favorable regulatory environment are fueling the market growth in the region. Meanwhile, Europe and Asia Pacific emerged as highly lucrative markets aided by surging healthcare investments and increasing rate of diagnosis of allergic disorders.
Manufacturers are focused on advancing their product portfolio through innovations to gain competitive advantage. The key players are investing in the development of next-generation autoinjectors with pediatric doses and features like voice instructions and user-friendly design. Market players are resorting to strategic collaborations and partnerships with service providers to enhance their presence across different geographies.
Figure 1. Global Epinephrine Autoinjector Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Epinephrine Autoinjector Market- Drivers
- Growing prevalence of anaphylaxis: The prevalence of life-threatening allergies such as food allergies and insect stings have been steadily rising across the world over the past few decades, posing a serious health concern. According to recent studies by organizations like World Allergy Organization data in 2021, approximately 500 million people globally suffer from potentially serious allergic conditions. If recent trends continue unabated, by the year 2050, over 50% of the population is projected to suffer from one or more allergic diseases. The impact of increasing allergy rates is already evident on healthcare systems and resources. Anaphylaxis reactions triggered by foods, medications or stings can be fatal if not addressed urgently through emergency medication like epinephrine injections. This has led to increase in demand for epinephrine auto-injectors which provide pre-filled doses of epinephrine to be administered during an allergic reaction. According to the data pulished by the U.S. National Institute for Health in 2021, over 400,000 individuals received emergency treatment for anaphylaxis in the U.S. alone in 2020 signifying a rise of nearly 25% from previous years. Epinephrine auto-injectors have become crucial lifesaving devices for people at risk of anaphylaxis.
- Rising Prevalence of Food Allergies: Rising prevalence of food allergies across global populations is a key driver behind the growth of the epinephrine autoinjector market. According to recent data published by Food Allergy Research & Education (FARE), 32 million Americans suffer from food allergies. This represents a significant increase over previous estimates and indicates that food allergies have been growing steadily each year. Similarly, research by the National Health and Medical Research Council (NHMRC) in Australia showed that around 4-6% of children and 1-2% of adults in that country have a diagnosed food allergy. Autoinjectors allow patients to self-administer epinephrine via an easy to use prefilled syringe mechanism, as opposed to relying on syringes or vials that require assembly during an emergency reaction. The convenience and ease of use of autoinjectors has made them a vital treatment option for the millions of people now living with food allergies globally.
Global Epinephrine Autoinjector Market- Opportunities
- Expanding into emerging markets: Expanding into emerging markets holds immense promise for growth in the epinephrine autoinjector market. Many large and populous developing nations are experiencing increasing prevalence of allergies andaphylaxis conditions triggered by environmental or food-related factors. However, access to emergency treatment devices remains limited in these underserved regions. Taping into the needs of such fast-developing healthcare sectors can open up revolutionary opportunities.
- Development of needle-free injection technologies: The development of needle-free injection technologies could provide a major opportunity in the epinephrine autoinjector market. Needle-free options would help alleviate many patients' fears surrounding typical injections containing needles. This is especially true for those requiring epinephrine autoinjectors, like individuals with severe allergies. Not having to use a needle would make epinephrine administration less anxiety-provoking during allergen exposure. The adoption of needle-free technologies for administering epinephrine could enhance the willingness of at-risk patients to promptly use the treatment during reactions, potentially resulting in further increases in treatment rates. The overall accessibility and acceptability of lifesaving allergy treatments would be greatly enhanced as a result.
Global Epinephrine Autoinjector Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.74 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 8.8% | 2032 Value Projection: | USD 4.95 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Mylan, Teva Pharmaceutical, Impax Laboratories, Adamis Pharmaceuticals Corporation, Pfizer, ALK Abello, Lincoln Medical, Hospira, Sanofi, Kaleo |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Epinephrine Autoinjector Market- Trends
- Adoption of digital health platforms for autoinjector training: The significant impact of incorporating digital health platforms for training on the utilization of epinephrine autoinjectors is driving notable changes in the epinephrine autoinjector market. Many pharmaceutical companies that manufacture epinephrine autoinjectors are developing digital training modules and apps to educate patients about how to identify the symptoms of severe allergic reactions and properly administer treatment. These digital trainings provide step-by-step instructions through videos and interactive simulations of how to use specific brands of autoinjectors. Patients can complete the self-paced online courses at their convenience.
- Increase in self-administration of epinephrine: The trend of self-administration of epinephrine by patients and caregivers during anaphylaxis has been steadily increasing over the past few years. More patients and caregivers are becoming aware of the life-saving benefits of early epinephrine administration for treating allergic reactions. This has led to a shift towards easy-to-use epinephrine auto-injectors that does not require prior training and can be easily self-administered even during anxious situations like anaphylaxis emergencies. This increasing demand is compelling more pharmaceutical manufacturers to develop advanced new-age anaphylaxis treatment devices, thus continuously expanding the size of the global epinephrine autoinjector market.
Global Epinephrine Autoinjector Market - Restraints
- High prices of branded devices: The high prices of branded epinephrine autoinjectors are significantly restraining the growth of the overall epinephrine autoinjector market. Epinephrine autoinjectors are life-saving devices used for emergency treatment of severe allergic reactions also known as anaphylaxis. However, the exorbitantly high prices of branded autoinjectors like EpiPen by Pfizer are putting them out of reach for many patients who need them. The high branded device prices are also restricting healthcare organizations and schools from stocking adequate quantities of epinephrine autoinjectors. According to the study published in Journal of Allergy and Clinical Immunology in 2021, almost 25% of schools surveyed in Chicago cited high drug costs as a barrier for stocking epinephrine autoinjectors. With more affordable generic alternatives becoming available in recent years, the market has witnessed gradual shift towards lower-priced options. However, the overall market opportunity remains under-tapped as long as availability and affordability issues persist due to exorbitant branded device pricing.
- Stringent regulations: Stringent regulations on the epinephrine autoinjector market by several government regulatory bodies are indeed restraining the growth potential of this sector. The approval process for new autoinjector devices and drugs has become very lengthy and complex in recent times. Several requirements have been added by authorities like the FDA in the U.S. and EU regulators regarding safety, efficacy and quality standards that manufacturers need to fulfil. This has significantly increased the time, cost and risk involved in bringing new products to the market. As a result, many smaller companies and startups are finding it prohibitive to enter this domain and compete with large established players. Lengthy clinical trials, rigorous testing and long review periods before approvals are delaying the launch of improved and innovative products.
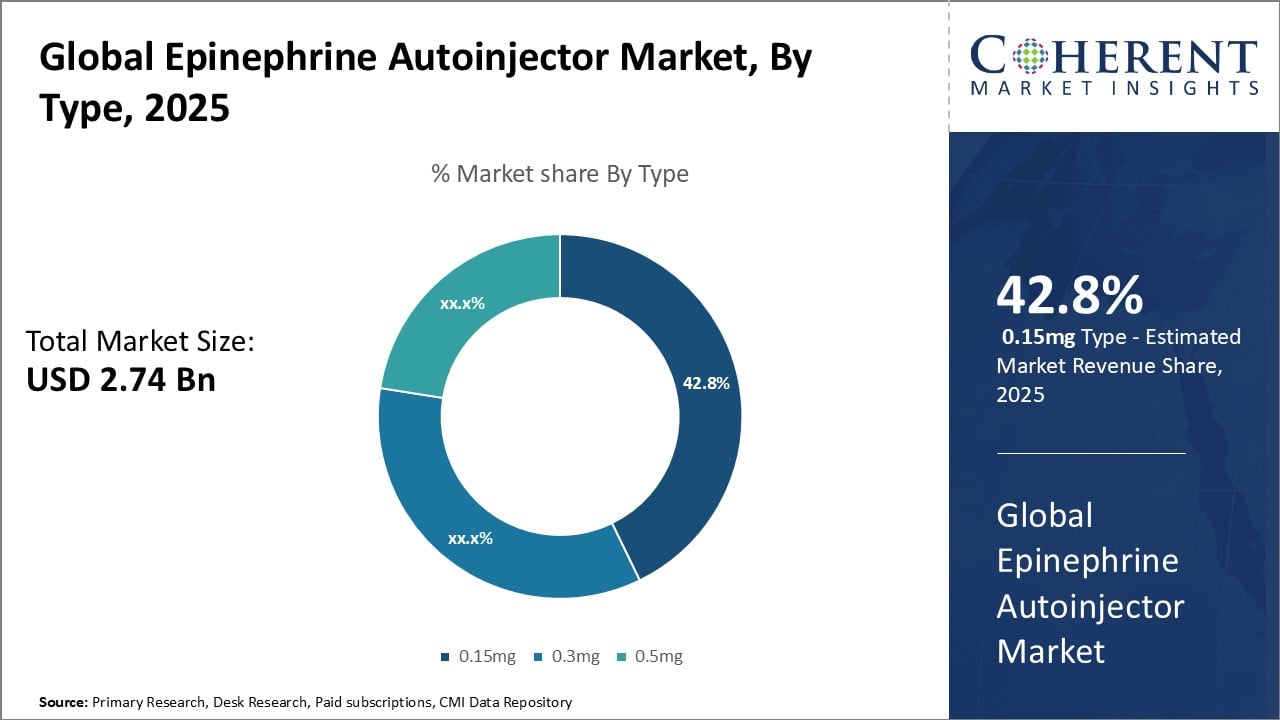
Figure 2. Global Epinephrine Autoinjector Market Share (%), By Type, 2025
To learn more about this report, Download Free Sample
Global Epinephrine Autoinjector Market- Recent Developments
Business Development Activities by the Market Players
- On September 20, 2023, the U.S. Food and Drug Administration declined to approve an alternative to epinephrine autoinjectors such as EpiPens, telling the maker of a nasal spray that more study is required. ARS Pharma, a pharmaceutical company that produces the drug, announced that the U.S. FDA had asked for a study to be completed. This study is to evaluate the effects of multiple doses of their spray, known as neffy, in comparison to multiple doses of an epinephrine injection.
- On August 10, 2023, Illinois Senate Democrats announced that it has enacted US$ 60 price cap on epinephrine autoinjector two-packs. The cap comes amid an ongoing injector shortage and follows similar moves in other states.
- In November, 2021, Pfizer, an American multinational pharmaceutical and biotechnology corporation coordinated with U.S. FDA to extend the expiration dates of specific lots of EpiPen 0.3 mg Auto-Injectors and its authorized generic version after review of stability data. This announcement is based on a careful review of product stability data provided by Pfizer.
Top companies in Global Epinephrine Autoinjector Market
- Mylan
- Teva Pharmaceutical
- Impax Laboratories
- Adamis Pharmaceuticals Corporation
- Pfizer
- ALK Abello
- Lincoln Medical
- Hospira
- Sanofi
- kaleo
Definition: An epinephrine autoinjector is a medical device that os designed for the rapid and convenient administration of epinephrine (adrenaline) during emergency situations such as severe allergic reactions (anaphylaxis). The device is pre-filled with a specific dose of epinephrine, allowing individuals, including those with known allergies, to self-administer the life-saving medication promptly. Epinephrine acts as a bronchodilator, narrowing blood vessels, and increasing blood flow to counteract severe allergic reactions. Compact and user-friendly, the autoinjector is crucial in providing quick and effective intervention, potentially preventing life-threatening outcomes associated with anaphylactic shock.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




