Global Epilepsy Monitoring Devices Market Size and Trends
Global epilepsy monitoring devices market is estimated to be valued at USD 576.2 Mn in 2025, and is expected to reach USD 833.2 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 5.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Increasing number of epilepsy cases globally can boost demand for advanced medical devices for epilepsy monitoring and diagnosis. Continuous technological advancements in medical devices industry has led to development of more accurate and easier to use epilepsy monitoring devices. Portable and wearable monitoring devices are gaining popularity among patients and healthcare providers due to benefits such as enhanced patient mobility and remote monitoring capabilities. Adoption of digital healthcare solutions and integration of Internet of Things (IoT) in medical devices is also expected to support the market growth during the forecast period. However, high costs associated with sophisticated epilepsy monitoring devices may hamper the market growth.
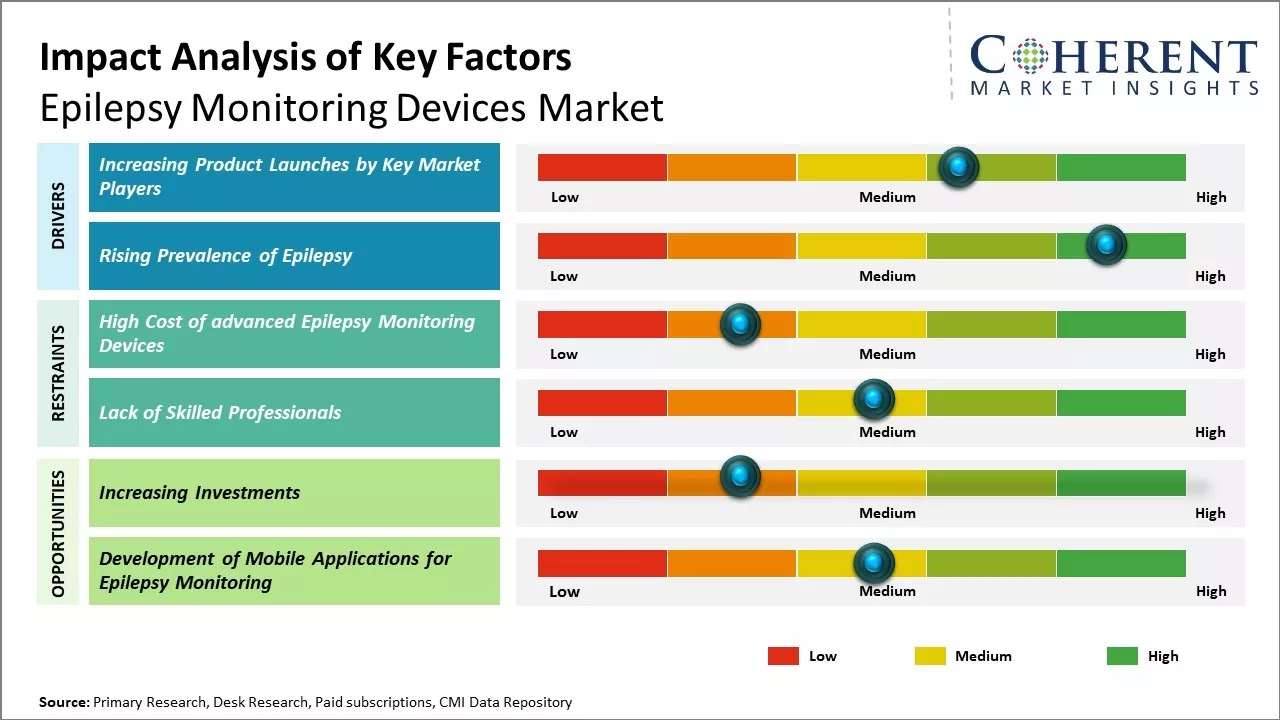
Market Driver – Increasing Product Launches by Key Market Players
Increasing adoption of organic growth strategies such as product launches by key market players is expected to drive the market growth over the forecast period. For instance, in October 2023, Ceribell, Inc., a medical technology company, launched ClarityPro, an AI-based software algorithm within the Ceribell EEG system that analyzes EEG waveforms to diagnose electrographic status epilepticus (ESE). ClarityPro algorithm provides clinicians with unprecedented abilities to quickly and accurately detect, diagnose, and monitor electrographic status epilepticus in critically ill patients.
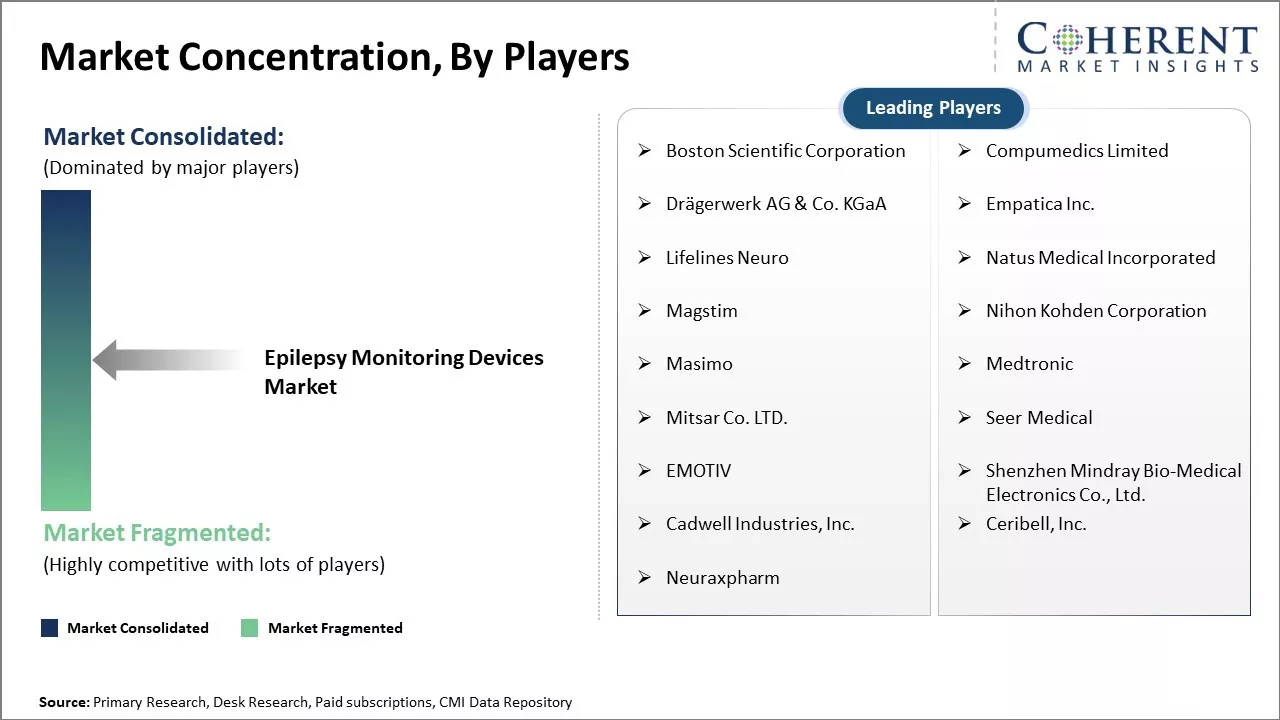
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Rising Prevalence of EpilepsyOne of the major drivers of the global epilepsy monitoring devices market is rising prevalence of epilepsy globally. Epilepsy is a chronic noncommunicable disease of the brain that affects people of all ages. However, it is more common in children and older adults. For instance, according to the data published by the World Health Organization, an estimated 5 million people are diagnosed with epilepsy each year globally.

To learn more about this report, Download Free Sample
Market Challenges – Lack of Skilled ProfessionalsThe lack of skilled professionals is a major challenge restraining the growth of the global epilepsy monitoring devices market. Epilepsy is a complex neurological condition that requires advanced expertise for effective diagnosis and management. However, there is a shortage of neurologists and epileptologists worldwide who have specialized training in epilepsy care. The use of advanced epilepsy monitoring devices can improve detection and treatment of seizures. However, the skills to interpret electroencephalogram readings and other diagnostic data gathered from these devices requires specialized postgraduate training, which lack in many parts of the world. Even in countries with adequate numbers of neurologists and availability of technology, there is often a lack of trained technicians and nurses who can assist physicians and expand epilepsy care programs. Unless more trained professionals are available worldwide through focused educational initiatives, many regions will not be able to take full advantage of new diagnostic and treatment options emerging in the epilepsy monitoring devices market. This shortage of skilled workforce is a major barrier negatively impacting the market growth.
Market Opportunities – Increasing Investments
Increasing investments for the development of epilepsy monitoring devices is expected to offer the lucrative opportunities in the market. For instance, in November 2021, Neurava, a startup located in the U.S., announced that it had received more than US$ 650,000 in seed funding for the development of wearable device to monitor and alert for the impending risk of sudden, unexpected death in epilepsy (SUDEP). The funding was led by Elevate Ventures with participation from Purdue Foundry, First Leaf Capital, UCB Biopharma, iO Life Ventures and angel investors.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
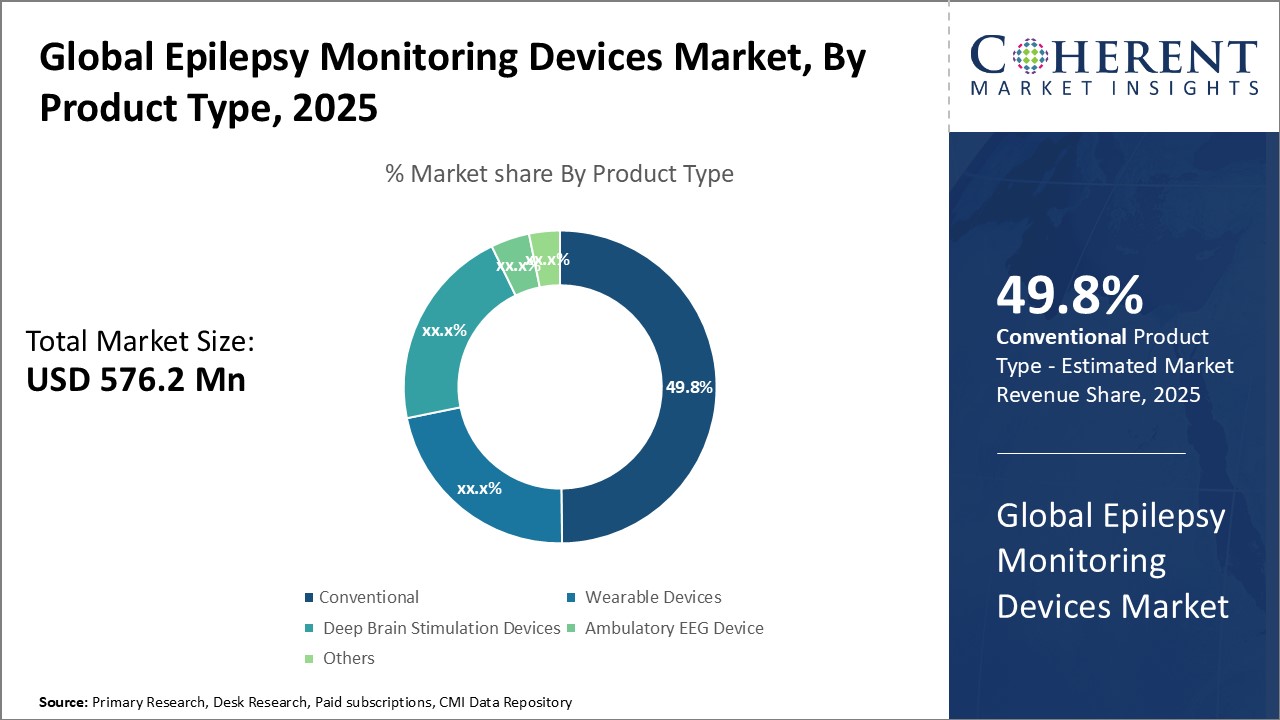
Insights, By Product Type: Wide Acceptance and Adoption of These Traditional Monitoring SolutionsProduct Type segment is sub-segmented into conventional and wearable devices, deep brain stimulation devices, ambulatory EEG device, and others. Conventional and wearable devices segment is expected to hold 49.8% of the market share in 2025 due to wide acceptance and adoption of these traditional monitoring solutions. Conventional devices such as electroencephalography (EEG) machines are used for epilepsy diagnosis and monitoring for decades. Their proven effectiveness and reliability have helped build strong brand loyalty among healthcare professionals. Conventional EEG devices offer versatility as their wired setup allows both inpatient and outpatient usage flexibility. Hospitals extensively rely on these conventional systems for round-the-clock epilepsy monitoring of admitted patients.
Insights, By End User: In-house infrastructure of Hospitals
End User segment is sub-segmented into hospitals, diagnostic centers, specialty clinics, and others. The hospitals segment is expected to hold 57.7% of the market share in 2025, owing to diverse factors. Hospitals serve as the primary point of diagnosis, treatment, and management for epilepsy patients worldwide. For conducting epilepsy monitoring tests, hospitals are equipped with dedicated monitoring units containing high-end conventional EEG equipment in their neurology and epilepsy departments. This large in-house infrastructure allows hospitals to handle both simple as well as complex epilepsy evaluations for thousands of admitted patients annually. Moreover, hospitals employ epileptologists and EEG technologists to efficiently operate the monitoring facilities and interpret test results. Reimbursements for epilepsy diagnostics are also favorable when conducted in hospitals. These advantages have helped hospitals emerge as the preferred and most accessible end users for both patients and referring physicians. With growing epilepsy prevalence globally, the demand for such specialized hospital services is growing steadily. Even with the rise of ambulatory testing options, hospitals are likely to maintain their strong position as the centralized long-term care providers for epilepsy.
Regional Insights
Need a Different Region or Segment? Download Free Sample
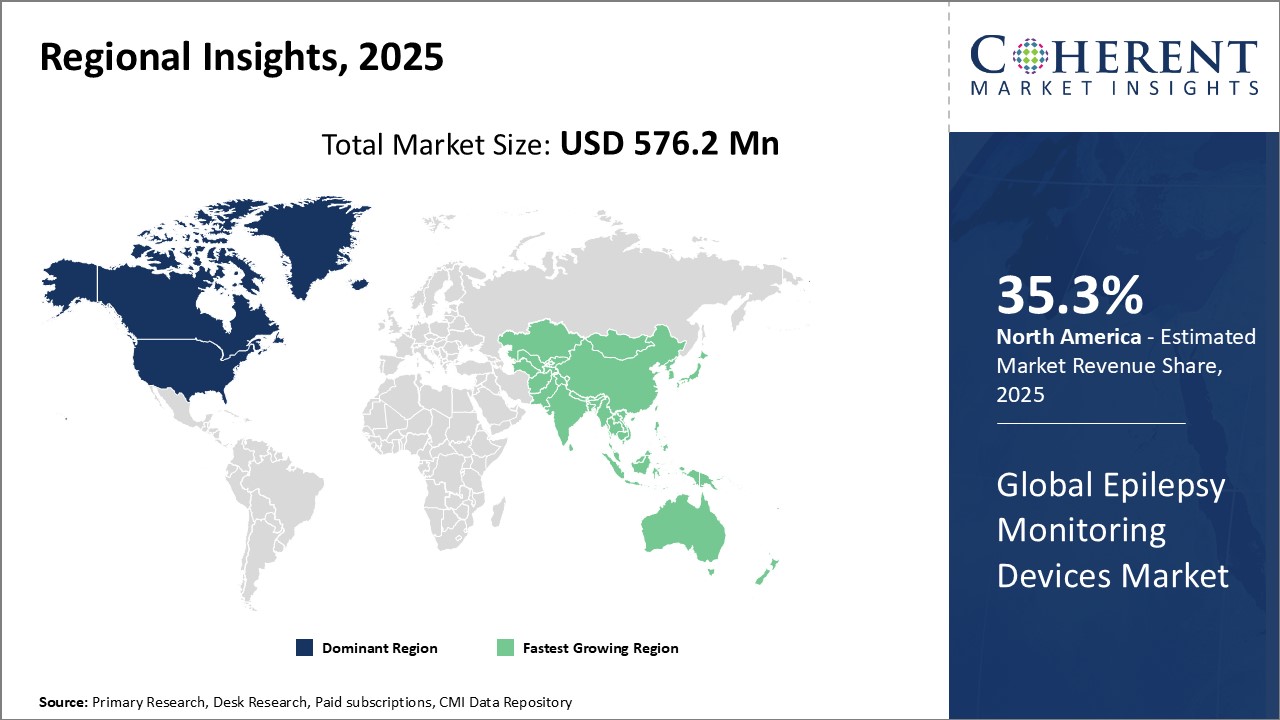
North America remains the dominant region in the global epilepsy monitoring devices market and is anticipated to hold 35.3% of the market share in 2025. Presence of key players like Medtronic, Natus Medical Incorporated, and Nihon Kohden, headquartered in U.S. and Canada. These companies have been investing heavily in R&D to develop advanced products. Moreover, North America is an early adopter of new technologies owing to higher awareness about epilepsy and healthcare expenditure. The favorable insurance and reimbursement policies also support the growth of expensive medical devices in the region.
The Asia Pacific region has been witnessing steady growth and is emerging as the fastest growing regional market for epilepsy monitoring devices market. The region has been witnessing steady economic growth with rising health expenditures. Countries like China and India have emerged as lucrative markets offering huge untapped growth potential. Major key market players have started establishing local manufacturing plants and distribution partners to capitalize on this opportunity. Additionally, the government has implemented various programs to spread awareness about epilepsy and make treatment affordable. This has boosted the demand for medical devices in the region. Asia Pacific represented one of the fastest growing regional markets driven by both domestic demand and exports within the Asia Pacific countries. The future prospects appear bright as healthcare infrastructure and standards continue to improve across nations.
Market Report Scope
Global Epilepsy Monitoring Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 576.2 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.4% | 2032 Value Projection: | USD 833.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Boston Scientific Corporation, Compumedics Limited, Drägerwerk AG & Co. KGaA, Empatica Inc., Lifelines Neuro, Natus Medical Incorporated, Magstim, Nihon Kohden Corporation, Masimo, Medtronic, Mitsar Co. LTD., Seer Medical, EMOTIV, Shenzhen Mindray Bio-Medical Electronics Co., Ltd., Cadwell Industries, Inc., Ceribell, Inc., Neuraxpharm |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Epilepsy Monitoring Devices Industry News
- On February 29, 2024, Empatica Inc., a healthcare company, launched EpiMonitor, an epilepsy monitoring system for adults and children aged 6 and above. EpiMonitor is an advanced wearable technology that is designed to detect generalized tonic-clonic seizures with high accuracy.
- In October 2022, Neuraxpharm, a global healthcare company, announced that it had signed a commercialisation agreement for mjn-SERAS, a wearable medical device that aims to predict the risk of having an epileptic seizure, with mjn-neuro., a manufacturer of medical devices with the aim of improving people's quality of life. With this agreement, Neuraxpharm increases its end-to-end offering for patients with epilepsy and enters the beyond-the-pill solutions market.
- In September 2021, The National Institutes of Health awarded a US$ 12.25 million grant to the University of California San Diego to develop and enhance brain-sensing and brain-stimulating platform technologies to enable treatment of drug-resistant epilepsy
*Definition: Epilepsy monitoring devices involves diverse medical devices used to record seizures and diagnose epilepsy in patients. This includes devices such as conventional video EEG systems, ambulatory EEG systems, and intraoperative monitoring devices that help epileptologists precisely monitor physiological brain signals and detect even subtle changes in brain activity that can indicate seizures. Advanced devices with real-time EEG monitoring, MRI compatibility, and wireless data transmission capabilities have further enhanced the field of epilepsy treatment and research globally.
Market Segmentation
- Product Type Insights (Revenue, USD Mn, 2020 - 2032)
- Conventional and Wearable Devices
- Deep Brain Stimulation Devices
- Ambulatory EEG Device
- Others
- End User Insights (Revenue, USD Mn, 2020 - 2032)
- Hospitals
- Diagnostic Centers
- Specialty Clinics
- Others
- Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Boston Scientific Corporation
- Compumedics Limited
- Drägerwerk AG & Co. KGaA
- Empatica Inc.
- Lifelines Neuro
- Natus Medical Incorporated
- Magstim
- Nihon Kohden Corporation
- Masimo
- Medtronic
- Mitsar Co. LTD.
- Seer Medical
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
