Global Barrett’s Esophagus Market Size and Trends
Global Barrett’s esophagus market is estimated to be valued at USD 5.32 Bn in 2025 and is expected to reach USD 7.70 Bn by 2032, growing at a compound annual growth rate (CAGR) of 5.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market is expected to witness positive growth over the forecast period due to rising geriatric population worldwide who are more prone to gastroesophageal reflux disease (GERD) and subsequent development of Barrett’s esophagus. Additional factors such as increasing obesity rates, growing adoption of unhealthy lifestyles and rising smoking prevalence are also expected to drive the risk of GERD, and fuel the market growth. However, lack of symptoms in early stages of Barrett’s esophagus may limit the market size potential to a certain extent.
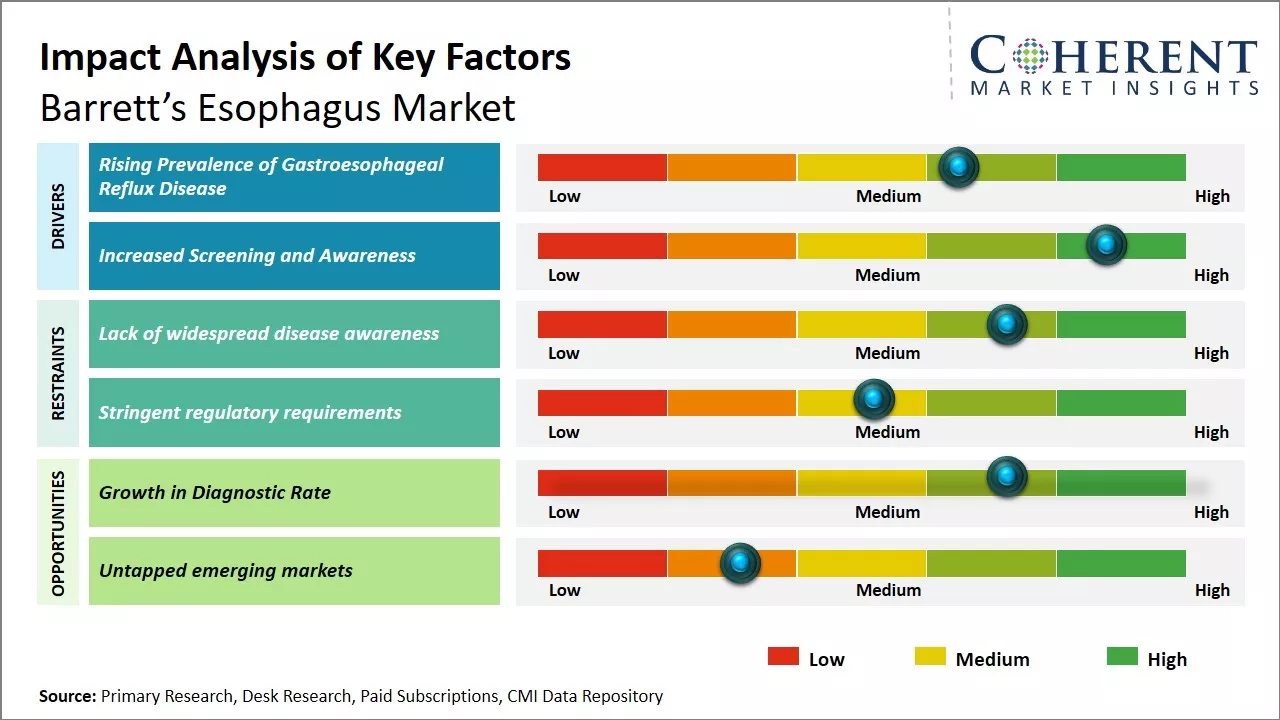
Rising Prevalence of Gastroesophageal Reflux Disease
One of the key drivers for growth of the global Barrett's esophagus market is increasing prevalence of gastroesophageal reflux disease (GERD) across global populations. GERD is a common condition that affects the digestive system, and causes a burning sensation in the chest or throat known as acid reflux. Long term or frequent reflux increases the risk of damage to the esophageal lining over time and may result in Barrett's esophagus.
Various lifestyle and dietary habits have contributed to higher instances of obesity and poor digestion. Consumption of fatty, spicy or acidic foods combined with lifestyle stress can weaken the lower esophageal sphincter, causing stomach acids to back up. This has led to a surge in GERD cases in recent decades. Changing demographics with aging populations also play a role as elderly individuals are more vulnerable to this condition. According to BMC Public Health Journal in 2023, globally, the prevalence of GERD grew significantly from 441.6 million to 783.9 million between 1990 and 2019, whereas the age-standardized prevalence rate increased little from 9,344.52 per 100,000 in 1990 to 9,574.45 per 100,000 in 2019.
Growing prevalence of GERD is a driver for Barrett's esophagus as prolonged reflux exposes the esophagus to damaging stomach acids over many years. This increases the chances of cellular changes developing in the esophagus lining. As lifestyle-related risk factors continue and populations age further, it is expected that the number of GERD patients will keep growing which will directly influence growth of Barrett's esophagus as well.
Market Concentration and Competitive Landscape

Get actionable strategies to beat competition: Download Free Sample
Increased Screening and AwarenessAnother major factor augmenting growth of global Barrett's esophagus market is greater efforts towards screening and diagnosis combined with raising awareness. Barrett's esophagus itself does not produce noticeable symptoms in early stages. As a result, many cases remain undiagnosed for long periods until complications appear. However, medical experts now recognize timely screening of high-risk groups as crucial for catching pre-cancerous changes early and improving treatment outcomes.

To learn more about this report, Download Free Sample
Market Challenges - Lack of widespread disease awareness:
One of the key challenges the market faces is lack of widespread disease awareness among at-risk populations and the general public. Since Barrett's esophagus often presents no overt symptoms, many cases go undiagnosed until the condition has progressed. Changes to lifestyle and diet can help reduce risk, but behaviors are difficult to modify without education. Treatment options are also limited, as surveillance and ablation methods are not always accessible or affordable. Further research is needed to develop more effective, non-invasive diagnostic and management solutions.
Market Opportunities - Growth in Diagnostic Rate:
The rising awareness and improving diagnostic capabilities have significantly increased the detection of Barrett's Esophagus over the past few years. Techniques such as high-resolution endoscopy and narrow-band imaging have made it possible to detect early changes in the esophagus that could indicate Barrett's Esophagus. As diagnostic rates go up, demand for drugs and devices targeted towards managing the condition also rises. Companies have a huge opportunity to cater to this growing patient pool by offering innovative treatment options.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
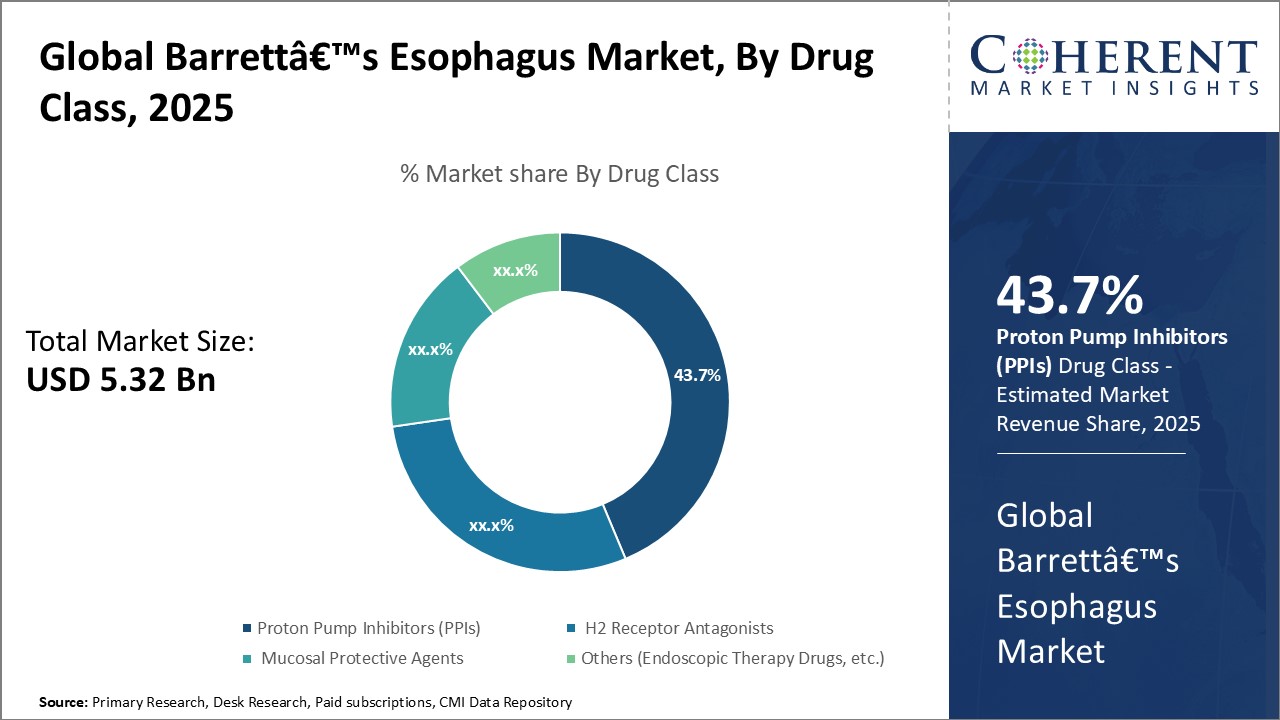
Insights, By Drug Class: proton pump inhibitors (PPIs) dominate due to efficacy against acid reflux
The drug class segment consists of proton pump inhibitors (PPIs), H2 receptor antagonists, mucosal protective agents, and others, out of which the PPIs contribute the largest share which is 43.7% in 2024. This is due to their strong efficacy in reducing gastric acid reflux. PPIs work by irreversibly blocking the gastric proton pumps located in the stomach's parietal cells, which are responsible for acid secretion. By suppressing acid production, PPIs provide powerful relief from acid reflux symptoms and allow the esophagus to heal.
PPIs are considered the standard first-line treatment for managing gastroesophageal reflux disease (GERD), the primary risk factor for Barrett’s esophagus. Clinical trials have demonstrated PPIs to be more effective than H2 receptor antagonists in treating GERD symptoms and healing erosive esophagitis. Their superior acid-suppressing capabilities enable PPIs to consistently control acid levels over a 24-hour period. This allows Barrett’s patients consistency in symptom relief and prevents further damage to the esophagus from reflux episodes.
PPIs are also very well-tolerated compared to other drug classes. These infrequently cause adverse effects and drug interactions. This high safety profile contributes to high levels of patient adherence, which is vital for Barrett’s management as it requires long-term acid suppression. PPIs offer once-daily dosing convenience as compared to H2 receptor antagonists that often require multiple daily doses. Comprehensive insurance coverage of branded and generic PPIs also improves accessibility and affordability for patients.
Insights, By Distribution Channel: hospital pharmacies are cornerstone of care for Barrett’s patients
The distribution channel includes hospital pharmacies, retail pharmacies, and online pharmacies, out of which the hospital pharmacies segment is anticipated to register 39.2% market share in 2025.This is primarily because Barrett’s esophagus is often diagnosed during inpatient hospital stays for upper endoscopy. Upon diagnosis, patients require intensive education from specialized nurses and pharmacists regarding their condition, prescribed treatment regimens, and risk of esophageal cancer.
Due to the severity of Barrett’s and need for close monitoring, hospitals perform follow-up endoscopies. During these procedures and subsequent outpatient visits with gastroenterologists, additional medications may be prescribed or existing therapies adjusted based on disease progression. Hospital pharmacies play a pivotal role in providing patients access to these specialized treatments immediately following changes to their regimens by physicians.
The complex drug therapies involved in long-term Barrett’s management, which typically include both PPIs and mucosal protective agents, also benefit from the support and expertise of hospital pharmacists. These can take time to counsel patients on intricacies like drug interactions, adverse effects to watch out for, and how to maintain optimal medication adherence over many years. This level of hands-on oversight is especially valuable for Barrett’s patients with comorbidities or at higher risk of disease complications.
Regional Insights
Need a Different Region or Segment? Download Free Sample
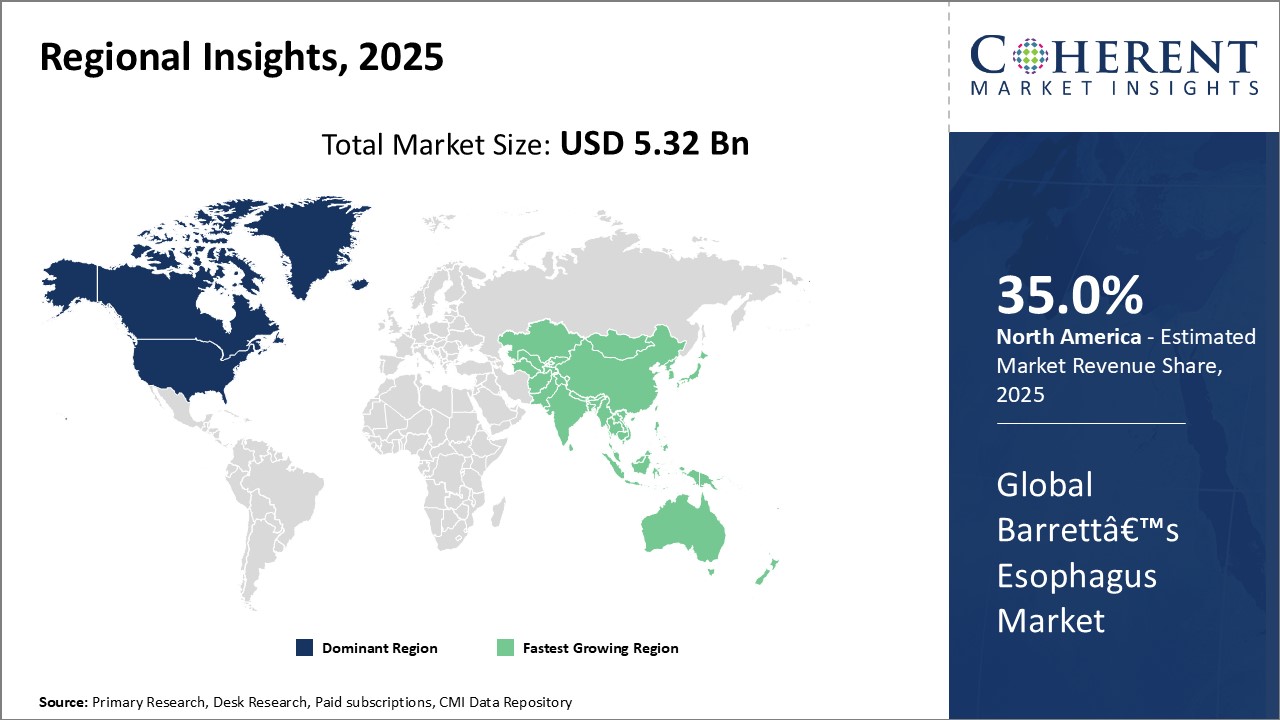
North America has been dominating the global Barrett’s esophagus market for past several years. The region accounts for over 35.0% share of the total market revenue owing to high prevalence of obesity, GERD and other risk factors associated with Barrett’s Esophagus in countries like U.S. Strong healthcare infrastructure and availability of advanced diagnosis and treatment options have enabled faster diagnosis and clinical management of patients in the region. Major market players have their headquarters in the region and focus on R&D to develop new drugs and devices. This has ensured early adoption of novel treatment options in North America.
The Asia Pacific region is emerging as the fastest growing market for Barrett’s esophagus currently. Countries like China, India and Japan are witnessing rapid economic development and improvement in healthcare access and quality of care. Rising healthcare expenditure, growing medical tourism and presence of generic drug manufacturers are driving growth. Further, growing obese population base and lifestyle changes making people prone to gastrointestinal disorders have widened the patient pool in the region. This has attracted many global industry players to tap into opportunities by establishing local manufacturing plants and distribution networks.
China alone contributes over one-third of the total Asia Pacific market value. This is majorly attributed to the country's huge population size whereby even lower prevalence of the disease translates into large number of patients. Rising disposable income is enabling people to opt for advanced diagnostic procedures and therapeutics. Both public and private healthcare sectors are expanding fast to cater the growing needs. This makes China one of the most promising markets offering high potential growth during the forecast period.
Market Report Scope
Global Barrett’s Esophagus Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 5.32 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 5.4% | 2032 Value Projection: | USD 7.70 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
AstraZeneca, Takeda Pharmaceutical Company Limited, Pfizer Inc., Novartis International AG, Sanofi, GlaxoSmithKline plc, Teva Pharmaceutical Industries Ltd., Dr. Reddy's Laboratories Ltd., Sun Pharmaceutical Industries Ltd., Lupin Limited, AbbVie Inc., Ferring Pharmaceuticals, Otsuka Pharmaceutical Co., Ltd., Taiho Pharmaceutical Co., Ltd., Ono Pharmaceutical Co., Ltd. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Barrett’s Esophagus Industry News
- In November 2023, Phathom Pharmaceuticals, Inc., a biopharmaceutical company that focuses on developing and commercializing novel treatments for gastrointestinal (GI) diseases, announced that the U.S. Food and Drug Administration (FDA) had approved VOQUEZNA (vonoprazan) tablets 10 mg and 20 mg, a novel potassium-competitive acid blocker (PCAB), as a new treatment for adults for the healing of all grades of Erosive Esophagitis, also known as Erosive GERD (gastroesophageal reflux disease), maintenance of healing of all grades of Erosive GERD, and relief of heartburn associated with Erosive GERD
- In March 2023, Hackensack Meridian JFK University Medical Center announced the launch of its new Gastroesophageal Reflux and Motility Program, which will provide expert diagnosis and treatment of gastroesophageal reflux disease, also known as GERD, and other related motility disorders that affect gastrointestinal muscles. GERD is characterized as nonerosive reflux disease (NERD), erosive esophagitis, and Barrett's esophagus.
- In January 2022, Dana-Farber Cancer Institute scientists published a study that demonstrated Barrett’s esophagus does not involve esophageal cells turning into intestinal cells, but stomach cells adopting some of the characteristics of intestinal cells. The second study, published in the current issue of Genes and Development, traces the series of molecular events by which this occurs.
- In March 2020, Cipla, a global pharmaceutical company, announced that it had received final approval for its Abbreviated New Drug Application (ANDA) for Esomeprazole for Oral Suspension 10mg, 20mg and 40mg from the United States Food and Drug Administration (US FDA). Cipla is the first company to file for the 10mg strength.
*Definition: Barrett's esophagus market covers products and therapies for detecting and treating Barrett's esophagus, a condition where the lining of the esophagus is damaged from acid reflux and gradually replaces by abnormal columnar epithelium cells. Products in this market include medication therapies to prevent acid reflux and decrease stomach acid production, endoscopy devices for screening and surveillance of Barrett's esophagus, biopsy tools for collecting tissue samples, and ablation devices used to remove pre-cancerous changes in the esophagus
Market Segmentation
- Drug Class Insights (Revenue, USD BN, 2020 - 2032)
- Proton Pump Inhibitors (PPIs)
- H2 Receptor Antagonists
- Mucosal Protective Agents
- Others
- Distribution Channel Insights (Revenue, USD BN, 2020 - 2032)
- Online Pharmacies
- Retail Pharmacies
- Hospital Pharmacies
- Regional Insights (Revenue, USD BN, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- AstraZeneca
- Takeda Pharmaceutical Company Limited
- Pfizer Inc.
- Novartis International AG
- Sanofi
- GlaxoSmithKline plc
- Teva Pharmaceutical Industries Ltd.
- Dr. Reddy's Laboratories Ltd.
- Sun Pharmaceutical Industries Ltd.
- Lupin Limited
- AbbVie Inc.
- Ferring Pharmaceuticals
- Otsuka Pharmaceutical Co., Ltd.
- Taiho Pharmaceutical Co., Ltd.
- Ono Pharmaceutical Co., Ltd.
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
