The global autoimmune polyglandular syndrome type 1 market size is expected to reach US$ 362.8 Mn by 2032, from US$ 289.1 Mn in 2025, exhibiting a CAGR of 3.3% during the forecast period.
Autoimmune polyglandular syndrome type 1 (APS1), also known as autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED), is a rare monogenic disorder caused by mutations in the autoimmune regulator (AIRE) gene.
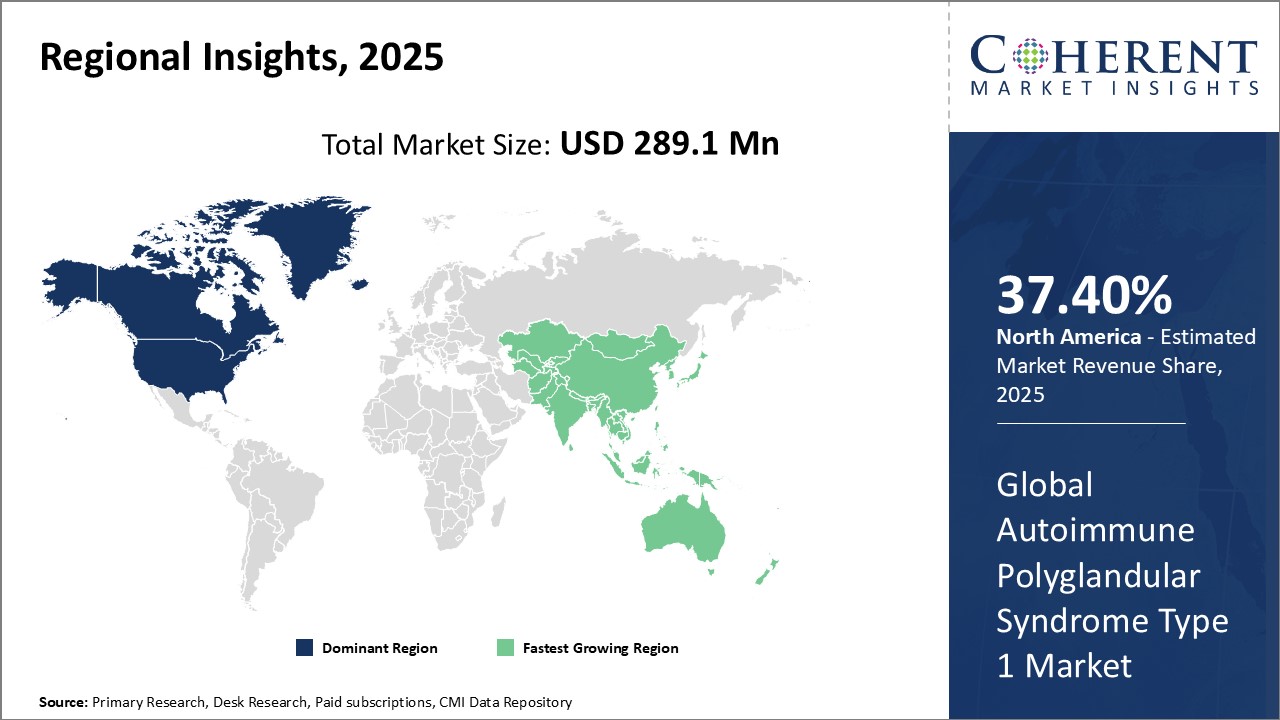
Global Autoimmune Polyglandular Syndrome Type 1 Market- Regional Insights
- North America is expected to be the largest market for autoimmune polyglandular syndrome type 1 during the forecast period, accounting for over 37.4% of the market share in 2025. North America has been the dominant region in the global autoimmune polyglandular syndrome type 1 market for several years. The sophisticated healthcare infrastructure and high adoption rate of advanced treatments are major factors influencing the large market size of the region. The U.S. accounts for the majority share of the market due to the presence of many key vendors and favorable regulatory guidelines regarding drug approvals. Additionally, growing research activities focusing on developing novel treatment therapies are providing steady growth opportunities in the region.
- The Europe market is the second-largest market with a share of 27.1% during the forecast period. This can be attributed to the presence of a strong reimbursement framework and favorable government initiatives for rare disease treatment. Awareness campaigns by advocacy groups regarding early diagnosis and management of autoimmune disorders are another factor propelling the European market. Additionally, approvals of orphan drugs for autoimmune polyglandular syndrome type 1 have augmented the sales volume in the region.
- Asia Pacific market is expected to be the third-largest market for autoimmune polyglandular syndrome type 1, accounting for over 21.2% of the market share in 2025. Rapidly developing economies like China and India are the major revenue generators for the region. Improving access to healthcare facilities and the rising medical tourism industry have enhanced the number of diagnosed cases in Asia Pacific over the past decade. Furthermore, increasing healthcare expenditures by various national governments have boosted the demand for high-priced specialty drugs. Several international players have also established their regional production units or tied up with local generic drug makers to capitalize on the high-volume opportunities in Asia Pacific.
Analyst View:
The autoimmune polyglandular syndrome type 1 market is expected to experience modest growth over the coming years. North America currently dominates due to rising awareness and timely diagnosis. However, Asia Pacific is likely to see faster uptake given the large patient pools in China and India. Drivers such as increasing research funding for rare diseases and growing diagnosis rates will support market expansion. Additionally, the development of new treatment techniques will drive adoption. However, lack of disease awareness in developing nations and high costs of care pose significant challenges for broader commercialization efforts.
Developing biomarkers for early detection will be crucial to improving clinical outcomes. Partnerships with academic research institutions can help accelerate the product pipeline. Favorable regulatory policies that expedite the review process for orphan drugs will incentivize investments. Manufacturers should focus on specialized treatments tailored for different disease stages. A multi-disciplinary treatment approach integrating different specialties can boost treatment effectiveness. Providers must work on building healthcare infrastructure in developing countries.
Collaboration within the medical community is important to streamline care coordination and management. Educating patients and the at-risk population will spur timely consultation. The future market outlook appears promising, provided ongoing R&D efforts address unmet needs and bring novel solutions.
Figure 1. Global Autoimmune Polyglandular Syndrome Type 1 Market Share (%), By Region, 2025
To learn more about this report, Download Free Sample
Global Autoimmune Polyglandular Syndrome Type 1 Market- Drivers
- Increased incidence of autoimmune disorders: The increased incidence of autoimmune disorders has led to higher diagnosis rates of autoimmune polyglandular syndrome Type 1 (APS1) over the past decade. APS1 is a rare and complex genetic disorder that results from mutations in the Autoimmune Regulator Gene (AIRE) gene. It is characterized by the simultaneous or sequential destruction of two or more endocrine glands due to autoimmune attacks. Various research studies show that the diagnosis rates of many autoimmune diseases, like type 1 diabetes, Addison's disease, hypothyroidism, etc., have risen significantly in the developed world. According to the Centers for Disease Control and Prevention (CDC) data from 2020, it is estimated that around 23.5 million Americans suffer from autoimmune diseases, which is nearly twice the number from 1999. As APS1 involves destruction of endocrine tissues, the rising incidence of underlying autoimmune conditions increases the risk of developing APS1. This has contributed to the growing demand for the diagnosis and treatment of APS1.
- Growing Genetic Research Insights: Advances in genetic research in recent years have provided valuable new insights into the underlying causes and pathogenic mechanisms of autoimmune polyglandular syndrome type 1 (APS1). Commonly known as APECED, this rare condition is caused by mutations in the AIRE gene that impair central tolerance and lead to a loss of immune tolerance against multiple endocrine tissues. Through large cohort studies and whole-exome sequencing efforts, researchers have mapped genetic and phenotypic variability associated with different AIRE mutations. This has expanded clinical understanding of the condition and allowed for the development of more accurate diagnostic criteria. Such growing scientific knowledge is fueling optimism around the future management and treatment of APS1. Researchers are leveraging genetic insights to develop tailored therapies that restore immune tolerance in affected tissues. Progress has already been made with hematopoietic stem cell transplantation, which shows promise for stabilizing disease.
Global Autoimmune Polyglandular Syndrome Type 1 Market- Opportunities
- Scope for development of new treatment drugs: There is currently a significant unmet need for effective treatment options for autoimmune polyglandular syndrome type 1 (APS1). APS1 is a rare genetic disorder characterized by the failure of multiple glands due to a loss of immune tolerance. Existing therapies focus on symptomatic treatment of individual gland failures as they occur, such as insulin therapy for diabetes or steroid replacement for adrenal insufficiency. However, there are no approved drugs that directly target the underlying autoimmune dysregulation driving multi-organ involvement in APS1. Developing new drugs that can restore immune tolerance and prevent disease progression could dramatically improve patients' quality of life and reduce lifelong medical costs associated with multi-organ management. Success in animal models and ongoing research on immune mechanisms open the door for clinical trials of immunomodulators, immunosuppressants, and other targeted therapies to profoundly alter the treatment landscape.
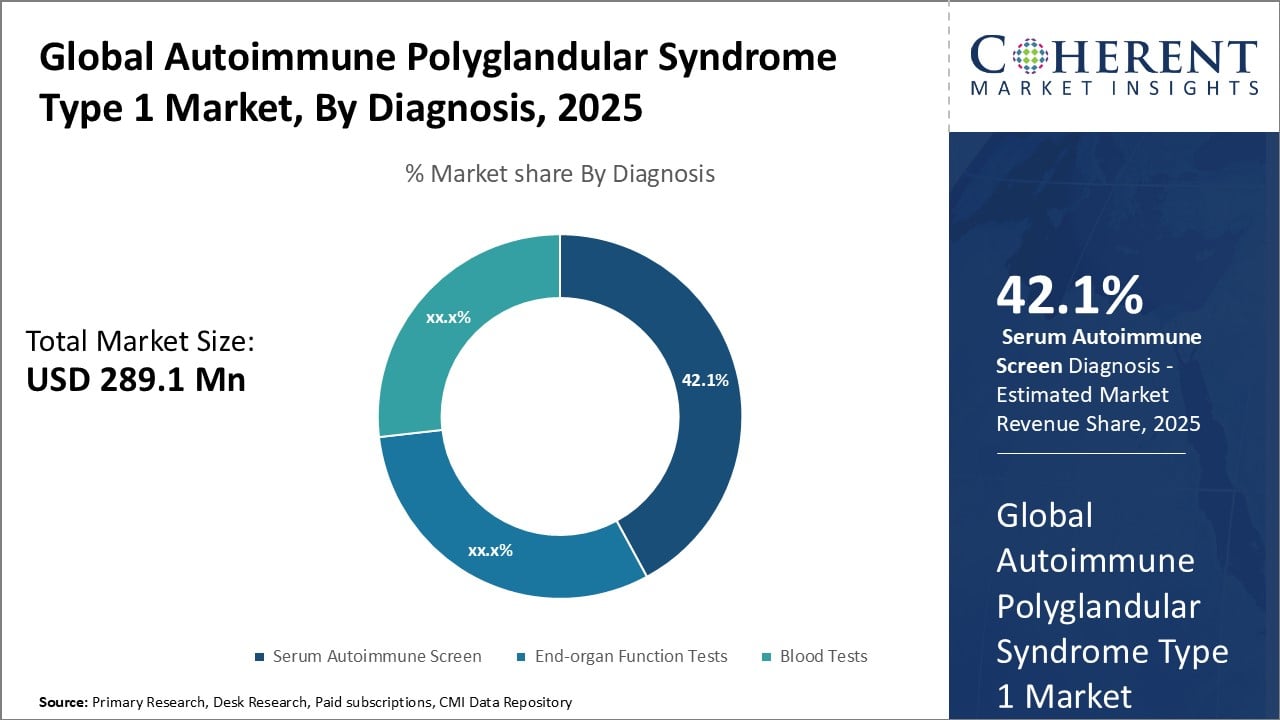
- Diagnostic kits and biomarkers: Diagnostic kits and biomarkers have huge potential to maximize growth in the autoimmune polyglandular syndrome type 1 market. This condition causes the immune system to attack and damage multiple glands, leading to serious health issues. However, a lack of standardized diagnostic criteria has made it difficult to accurately diagnose and manage the syndrome. Advanced diagnostic tools can help address this challenge. Novel biomarker discoveries are opening pathways to more precise diagnosis of autoimmune polyglandular syndrome type 1. For example, researchers have identified autoantibodies against interferon omega as potential biomarkers for the disorder. Ongoing research is exploring additional biomarkers in tissues, cells, and bodily fluids that can help distinguish this syndrome from related conditions. Widespread adoption of standardized diagnostic criteria and biomarkers has the potential to revolutionize the management of autoimmune polyglandular syndrome type 1 patients. This represents a significant growth driver for the overall market in the coming years.
Global Autoimmune Polyglandular Syndrome Type 1 Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 289.1 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 3.3% | 2032 Value Projection: | USD 362.8 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Pfizer Inc., GlaxoSmithKline plc, Novartis AG, Mylan N.V., Teva Pharmaceutical Industries Ltd., Sanofi, F. Hoffmann-La Roche Ltd., Zydus Cadila, Lupin, Amneal Pharmaceuticals LLC., Cipla Inc., Aurobindo Pharma, Glenmark Pharmaceuticals Limited, Eli Lilly and Company, Sun Pharmaceutical Industries Ltd., Allergan, Bristol-Myers Squibb Company, Takeda Pharmaceutical Company Limited, Abbott, and LEO Pharma A/S |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Autoimmune Polyglandular Syndrome Type 1 Market- Trends
- Precision and personalized medicine: The growing field of precision and personalized medicine is having a significant impact on the autoimmune polyglandular syndrome type 1 (APS1) market. APS1 is a rare genetic disorder that causes multiple endocrine gland failures. With advances in genetic testing and profiling, researchers are gaining a much deeper understanding of the molecular mechanisms behind APS1. By identifying specific gene mutations and variants associated with individual patients, doctors can develop highly tailored treatment plans based on a person's unique genetic profile and predicted disease progression. This represents a shift away from traditional one-size-fits-all therapies towards precise interventions designed for individual patients. Several biotech companies are now developing gene-targeted drugs that aim to suppress faulty immune responses at the genetic level in APS1. For example, Boston, U.S.-based Bit Bio, an award-winning spinout from the University of Cambridge recently launched a phase 2 trial of an IL-21 monoclonal antibody for APS1 caused by AIRE gene mutations (source: clinicaltrials.gov). By homing in on the specific dysfunctional immune pathways driving a person's APS1, these precision medicines have the potential for much higher response rates compared to traditional broad immunosuppressants.
- Stem cell therapy research: Recent advancements in stem cell therapy research have shown promising results for treating a variety of autoimmune conditions. This includes early phase clinical trials investigating the use of stem cells to treat autoimmune polyglandular syndrome type 1 (APS-1), a rare genetic disorder characterized by simultaneous dysfunction of multiple endocrine glands. In APS-1, the immune system mistakenly attacks and destroys insulin-producing cells in the pancreas and other hormone-producing glands. Conventional treatment focuses on replacing lost hormones and managing symptoms, but it does not cure the underlying immune dysfunction.
Global Autoimmune Polyglandular Syndrome Type 1 Market - Restraints
- Lack of awareness: Autoimmune polyglandular syndrome type 1 is a rare autoimmune disease that affects multiple endocrine glands. However,a lack of overall awareness about this condition among patients as well as healthcare providers has been a major factor hindering the growth potential of this market. Due to the rare nature of the disease and presence of non-specific symptoms in the early stages, proper and timely diagnosis remains a challenge. This ultimately leads to the progression of the disease to more advanced forms over time. According to a study published in the Orphanet Journal of Rare Diseases in 2021, about 63% of patients with autoimmune polyglandular syndrome type 1 in Germany suffered a delay in diagnosis of more than 5 years. Such delayed diagnoses result in reduced treatment effectiveness and increase lifelong dependence on hormone replacement therapies. Initiatives to educate people and train healthcare providers can help address this and support optimized growth of this niche market.
- High cost of treatment: The high cost of treatment is significantly hampering the growth of the autoimmune polyglandular syndrome type 1 market. Autoimmune polyglandular syndrome type 1 is a rare disorder that results from failure of immune system to recognize the body's own tissues. It requires lifelong treatment to manage the various conditions associated with it. However, the treatment process is very expensive, which many patients can't afford. The treatment depends on the symptoms presented in each patient but often involves hormone replacement therapy and immunosuppressive medications. Hormone replacement medicines like hydrocortisone, levothyroxine, etc. need to be taken throughout life, which costs thousands of dollars each year. Similarly, immunosuppressants have a high price tag. Both a lack of insurance schemes in low resource regions and high drug development costs pose major barriers to market expansion currently.
Figure 2. Global Autoimmune Polyglandular Syndrome Type 1 Market Share (%), By Diagnosis, 2025
To learn more about this report, Download Free Sample
Global Autoimmune Polyglandular Syndrome Type 1 Market- Recent Developments
Business Development Activities by the Market Players
- On July 7, 2023, the National Organization for Rare Disorders (NORD) and the APS Type 1 Foundation, a NORD member organization, announced the awarding of a US$50,000 grant for research into this rare autoimmune disorder. The grant, which is part of NORD’s Jayne Holtzer Rare Disease Research Grants Program, was made possible by funding from the APS Type 1 Foundation.
Top companies in Global Autoimmune Polyglandular Syndrome Type 1 Market
- Pfizer Inc.
- GlaxoSmithKline plc
- Novartis AG
- Mylan N.V.
- Teva Pharmaceutical Industries Ltd.
- Sanofi
- F.Hoffmann-La Roche Ltd.
- Zydus Cadila
- Lupin
- Amneal Pharmaceuticals LLC.
- Cipla Inc.
- Aurobindo Pharma
- Glenmark Pharmaceuticals Limited
- Eli Lilly and Company
- Sun Pharmaceutical Industries Ltd.
- Allergan
- Bristol-Myers Squibb Company
- Takeda Pharmaceutical Company Limited
- Abbott
- LEO Pharma A/S
Definition: Autoimmune polyglandular syndrome type 1 (APS-1), also known as autoimmune polyendocrine syndrome type 1 or Autoimmune Polyendocrinopathy Candidiasis Ectodermal Dystrophy (APECED), is a rare autoimmune disorder characterized by the coexistence of multiple endocrine and non-endocrine autoimmune diseases. APS-1 is caused by mutations in the AIRE (Autoimmune Regulator) gene.
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




