Genetic Toxicology Testing Market Size and Trends
The global genetic toxicology testing market is estimated to be valued at USD 1.60 Bn in 2025 and is expected to reach USD 3.83 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 13.3% from 2025 to 2032. The market is witnessing significant growth owing to strict safety regulations for new drug development and growing concerns over the toxicity of chemicals. Several developments such as growing contract research organizations and adoption of new technologies like next generation sequencing and cell imaging systems are helping drive the market growth. However, the high cost associated with genetic toxicology tests and lack of skilled professionals poses a major challenge for the market growth.
Discover market dynamics shaping the industry: Download Free Sample
The genetic toxicology testing market is expected to witness lucrative growth over the forecast period. Increasing investments by the pharmaceutical and biotechnology companies in drug discovery and development is creating the demand for genetic toxicology services. In addition, rising investments by governments worldwide to promote healthcare initiatives is also boosting the market growth. However, lengthy approval timeframes and processes continue to remain a key challenge for seamless market growth.
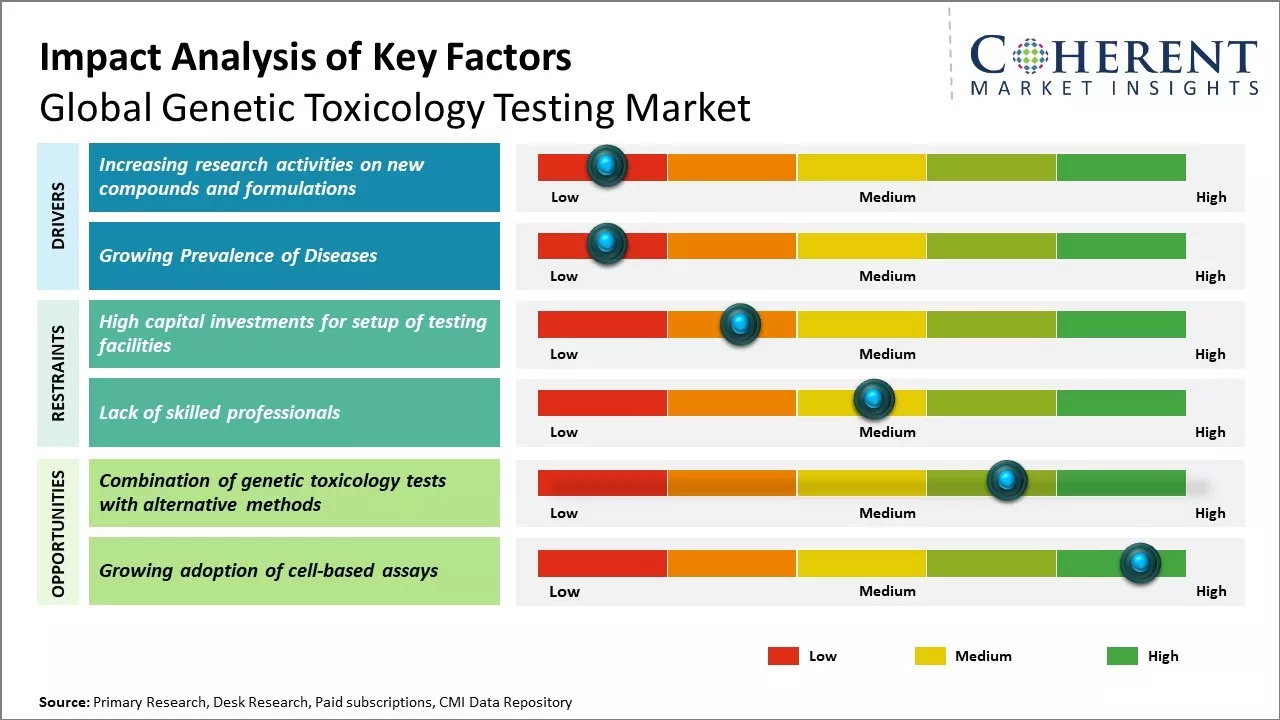
Increasing research activities on new compounds and formulations
Global genetic toxicology testing market has been witnessing strong growth due to increasing investments by major players in researching new formulations and novel compounds. Stringent regulations around drug safety and efficacy testing have prompted pharmaceutical companies to extensively evaluate new chemical entities for their potential to induce genetic damage. The COVID-19 pandemic has also highlighted the need for thorough pre-clinical examination of vaccines and therapeutics before human trials. There is a keen focus on developing advanced testing methods beyond traditional in vitro and in vivo assays. Areas such as human cell-based 3D tissue models, organ-on-chip microfluidic platforms and human stem cell-derived systems are being explored to improve predictability and translate genotoxic findings to humans early on. For example, the National Institutes of Health has funded several research projects over 2021-22 aimed at establishing human organotypic cultures incorporated with micro physiological readouts for genetic toxicology risk assessment. This is hoped to bridge existing gaps between animal and human responses. Moreover, the need to slash testing timelines and costs have fueled the adoption of rapid genomics and high-throughput screening approaches coupled with machine learning algorithms for big data analysis. For instance, in May 2022, Pfizer's establishment of a global drug development center at the IIT Madras Research Park in Chennai marks a significant consolidation of critical R&D capabilities. This center will focus on developing active pharmaceutical ingredients (APIs) and finished dosage forms (FDFs) for specialized products, including complex formulations, controlled-release dosage forms, device-combination products, lyophilized injections, powder-fill products, and ready-to-use formulations.
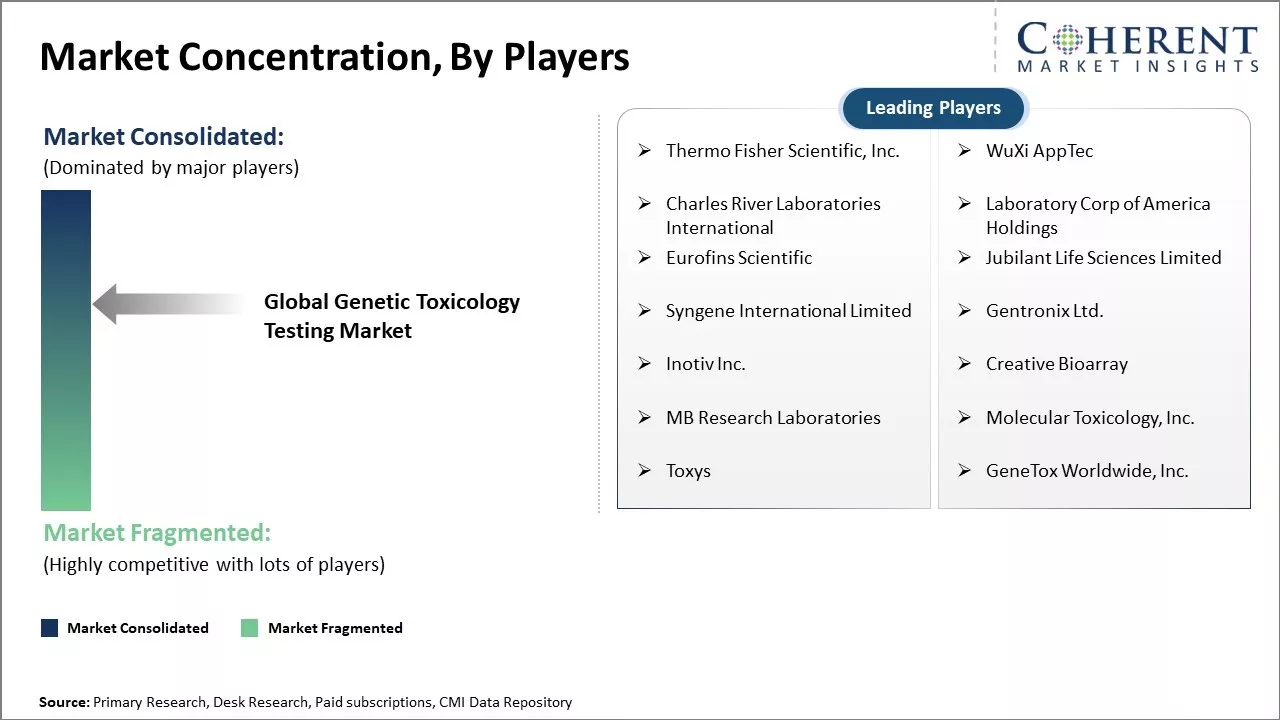
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Growing Prevalence of Diseases
With rising environmental pollution and changing lifestyles, the prevalence of chronic diseases like cancer has increased significantly all over the world. According to various studies, almost one in two men and one in three women worldwide develop cancer during their lifetime. Genetic factors play an important role in the development of many diseases. Understanding the genetic causes and mechanisms helps in early detection, prevention, and effective treatment of diseases. Growing awareness among people regarding genetics and diseases has propelled the demand for various genetic toxicology tests. These tests help assess the toxicity of various chemicals and agents on human genes. They are crucial in developing safer drugs and determining environmental and occupational hazards. Pharmaceutical companies extensively rely on genetic toxicology studies to ensure drug safety before clinical trials. Stringent regulations regarding drug development and approval processes have further boosted the need for such tests.
Key Takeaways from Analyst:
The global genetic toxicology testing market is expected to witness significant growth over the forecast period. Stringent regulatory mandates regarding drug safety are a key driver as they are necessitating genetic toxicology tests for new drug development. Growing R&D investment in the pharmaceutical and biotechnology industries will also contribute to the market growth. However, the high cost of genetic toxicology test kits and services may restraint market expansion to some extent.
North America will continue dominating the genetic toxicology testing market share due to the presence of many pharmaceutical companies and stringent drug approval regulations in the region. Asia Pacific offers lucrative opportunities for market players and will emerge as the fastest growing regional market. This can be attributed to increasing research activities by companies to strengthen their pipeline of drugs. Also, the trend of combination tests that can assess multiple toxic effects in a single assay will gain traction over the forecast period. Market players should also explore opportunities in emerging economies of Asia Pacific and Latin America given increasing investments in healthcare research in these regions.
Market Challenges: High capital investments for setup of testing facilities.
High capital investments required for setting up genetic toxicology testing facilities is posing a major challenge for the growth of the global genetic toxicology testing market. Establishing a dedicated genetic toxicology testing laboratory mandates significant capital investments ranging anywhere between US$5 and US$10 million dollars. This is a substantial amount for biotech and pharmaceutical companies, especially small and medium enterprises. Setting up such labs involves high costs associated with sourcing and installation of specialized equipment such as cell culture systems, genetic analyzers, bioinformatics solutions and other auxiliary infrastructure. Moreover, requisite qualified personnel with expert knowledge on genetic toxicology must be hired to efficiently operate and maintain such advanced testing facilities. This further adds to the operational expenses. The costs rise progressively with increasing scope and scale of operations. For instance, conducting in vivo animal testing on a large sample size requires dedicated animal housing with specialized care and handling facilities in accordance with global quality standards. This amplifies the initial setup costs substantially. Additional operational costs are also incurred on a continual basis for consumables, reagents, procurement, and feeding of the test subject animals as well as their veterinary care. Periodic equipment upgrades and customization as per evolving industry guidelines also demand recurring capital investments. Consequently, smaller companies and start-ups dealing with limited capital budgets find it extremely challenging to bear such heavy capital expenditures for instituting internal genetic toxicology testing capabilities.
Market Opportunities: Combination of genetic toxicology tests with alternative methods
The combination of genetic toxicology tests with alternative methods is significantly restraining the growth of the global genetic toxicology testing market. Advancements in alternative testing approaches such as in silico and in vitro techniques have allowed the reduction and replacement of animal testing. These alternative methods are providing faster, cost-effective, and more reliable results as compared to traditional animal-based assays. They are also ethical and minimize animal suffering. The adoption of alternative methods is increasing across industries as well as regulatory agencies. For example, the European Union Reference Laboratory for Alternatives to Animal Testing (EURL ECVAM) promotes alternative methods for evaluation of chemicals under the REACH and cosmetics legislation. They have recommended 15 alternative methods for replacing animal testing until now. Similarly, in the U.S., the Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) encourages implementing alternative methods for chemicals, biomedical devices and research purposes.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
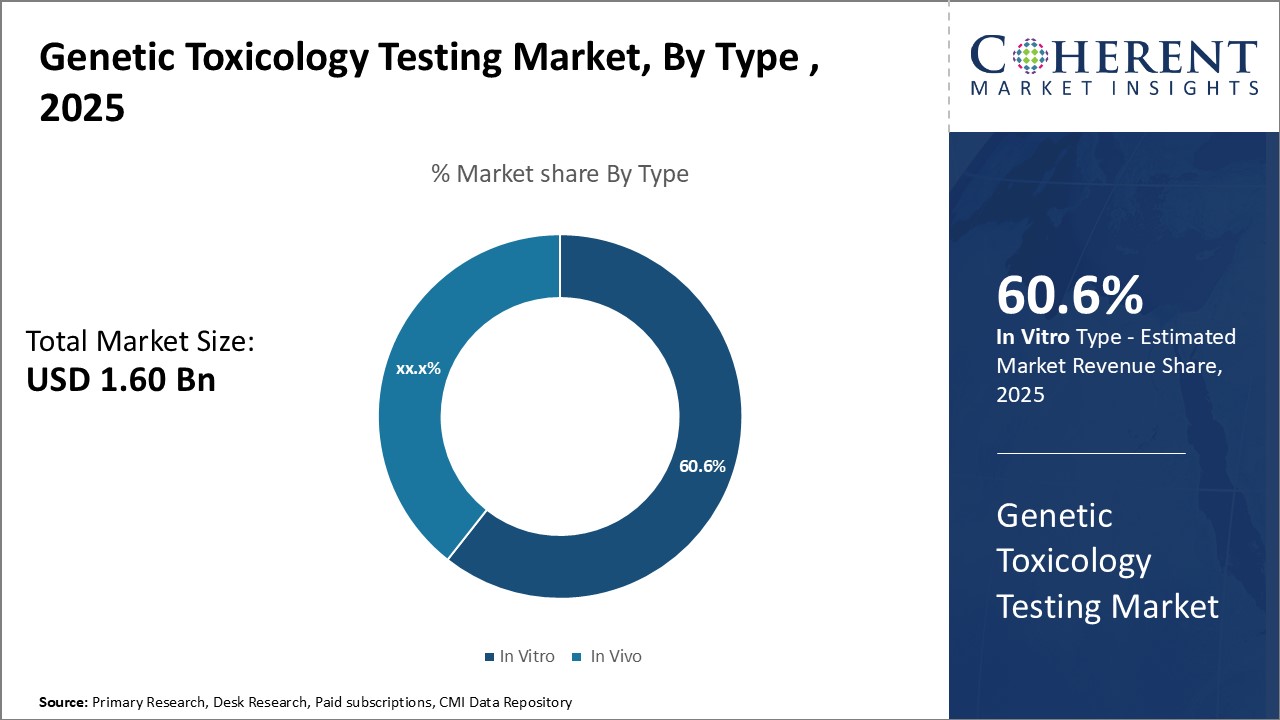
By Type - Rising Need for Cell-based Assays
In terms of type, in vitro is expected to contribute the highest share of the market, accounting for 60.6% of the market share in 2025 owing to the growing need for cell-based assays. Traditional in vivo testing methods are being replaced due to associated high costs and lengthy testing times. In vitro testing provides quicker results and reduces the use of live animals in experiments. Technological advancements have enhanced in vitro methods to closely mimic human in vivo conditions. 3D tissue cultures and microfluidic cell culture chips better represent physiological responses. This improves prediction of how chemicals may impact humans. Advancements in high-throughput screening also facilitate automated testing of thousands of chemicals simultaneously in vitro. This allows evaluating large chemical libraries being developed by pharmaceutical and chemical companies in a timely manner. High-throughput screening with robotic liquid handling has increased the efficiency and productivity of in vitro tests. Genetic toxicologists can screen more test articles in less time to speed up product development and ensure safety.
By Product - Rising Demand from Pharma Industry
In terms of product, reagents is expected to contribute the highest share of the market with 35.5% in 2025 due to the rising demand from the pharmaceutical industry. Genetic toxicology testing is widely conducted during drug development to ensure new molecules are safe. Pharmaceutical companies extensively use reagents like mutagens and consumables like cell lines to perform a battery of in vitro and in vivo tests on drug candidates. A variety of reagents catering to different test methods like Ames test, micronucleus assay, and chromosomal aberration analysis are consistently purchased. Stringent safety regulations enforced by regulatory bodies also drive the need for genetic toxicology testing in the pharma sector. Companies must demonstrate the genetic safety of molecules before they can be tested on humans. This fuels the consumption of a diverse range of reagents and consumables designed for each specific test protocol. As the pharma industry progresses novel drug entities, the demand for associated toxicology testing tools continues to grow.
By End User - Rising Outsourcing to CROs
In terms of end user, contract research organizations is expected to contribute the highest share of the market with 40.5% in 2025 owing to rising outsourcing of genetic toxicology testing. Pharmaceutical, chemical, and pesticide manufacturers increasingly rely on CROs to perform these tests due to associated high costs and specialized expertise. CROs can complete the work faster and more cost-effectively due to economies of scale and dedicated facilities. They also ensure compliance with global testing standards through accredited laboratories and trained personnel. Outsourcing also allows core companies to focus on their key competencies rather than non-core operations like toxicology screening. CROs provide a one-stop-shop for various in vivo and in vitro studies required during product development. This streamlines internal workflows. Additionally, working with global CROs opens new geographic markets for companies by helping navigate varying international regulations. Thus, rising R&D expenditures along with focus on core competencies have boosted genetic toxicology testing outsourcing to CROs.
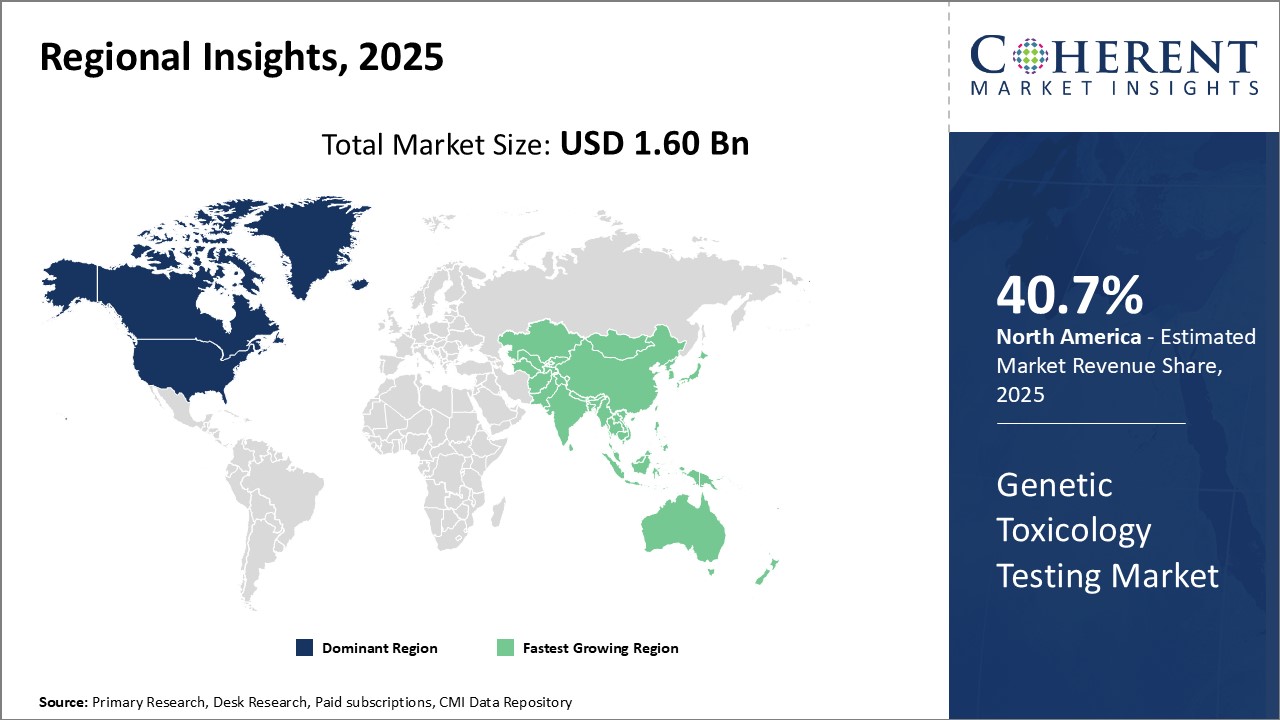
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America has established itself as the dominant region. The region is expected to account for a market share of 40.7% in 2025 in the global genetic toxicology testing market. With the presence of major pharmaceutical and biotechnology companies, the U.S. contributes significantly to the overall market size in the region. Stringent regulations regarding drug safety imposed by regulatory bodies such as FDA have pushed companies to invest heavily in genotoxicity screening at early stages of drug development. Additionally, the region is an attractive hub for outsourcing toxicology services due to availability of experienced professionals and established infrastructure for testing.
The Asia Pacific region is poised to be the fastest growing market for genetic toxicology testing globally. Rapid expansion of pharmaceutical and generic drug manufacturing industries in countries such as China, India, and South Korea is a major factor driving market growth. These countries contribute over 60.5% of global generics production and account for nearly 35.5% of USFDA-approved facilities located outside the U.S. With growing acceptance of outsourcing drug development activities to Asia Pacific, the demand for supporting preclinical services including genetic toxicology is surging rapidly. Governments in the region are also implementing stringent regulations modelled after international guidelines to assure drug safety, which necessitates the widespread adoption of genetic toxicology protocols.
Market Report Scope
Genetic Toxicology Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.60 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 13.3% | 2032 Value Projection: | USD 3.83 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Thermo Fisher Scientific, Inc., WuXi AppTec, Charles River Laboratories International, Laboratory Corp of America Holdings, Eurofins Scientific, Jubilant Life Sciences Limited, Syngene International Limited, Gentronix Ltd., Inotiv Inc., Creative Bioarray, MB Research Laboratories, Molecular Toxicology, Inc., Toxys, and GeneTox Worldwide, Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Segmentation
- Type Insights (Revenue, USD Bn, 2020 - 2032)
- In Vitro
- In Vivo
- Product Insights (Revenue, USD Bn, 2020 - 2032)
- Reagents
- Consumables
- Assays
- Services
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Contract Research Organizations
- Pharmaceutical Companies
- Chemical Companies
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Thermo Fisher Scientific, Inc.
- WuXi AppTec
- Charles River Laboratories International
- Laboratory Corp of America Holdings
- Eurofins Scientific
- Jubilant Life Sciences Limited
- Syngene International Limited
- Gentronix Ltd.
- Inotiv Inc.
- Creative Bioarray
- MB Research Laboratories
- Molecular Toxicology, Inc.
- Toxys
- GeneTox Worldwide, Inc.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
