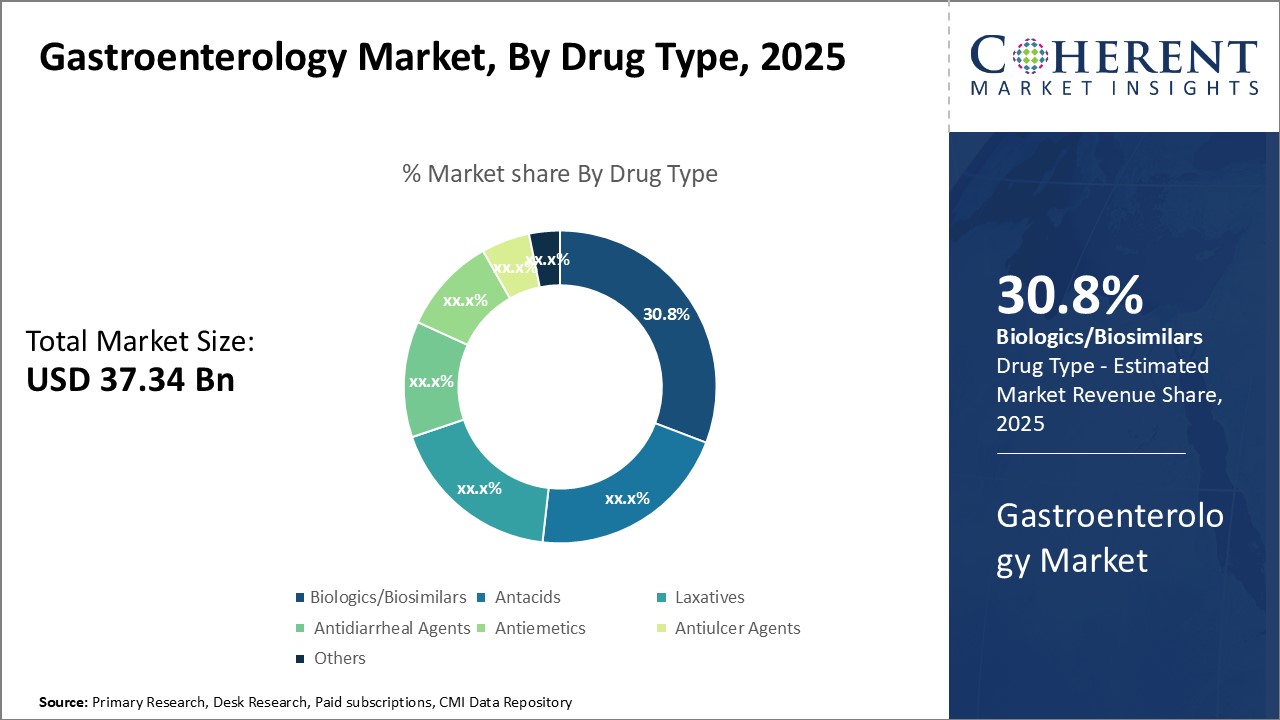
Global gastroenterology market is estimated to be valued at USD 37.34 Bn in 2025 and is expected to reach USD 57.30 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 6.3% from 2025 to 2032.
Key Takeaways:
- By Drug Type, Biologics/Biosimilars segment is estimated to contribute the highest market share of 30.8% in 2025, owing to their targeted mechanisms of action.
- By Dosage Form, Oral segment is estimated to contribute the highest market share of 42.3% in 2025, due to its convenience and ability to support patient compliance.
- By Disease Type, Inflammatory Bowel Diseases (IBD) segment is estimated to contribute the highest market share of 41.7% in 2025, owing to rising prevalence worldwide.
- By Distribution Channel, Hospital Pharmacies hold the largest market share in 2025 as Advancements in Biologic and Biosimilar Therapies.
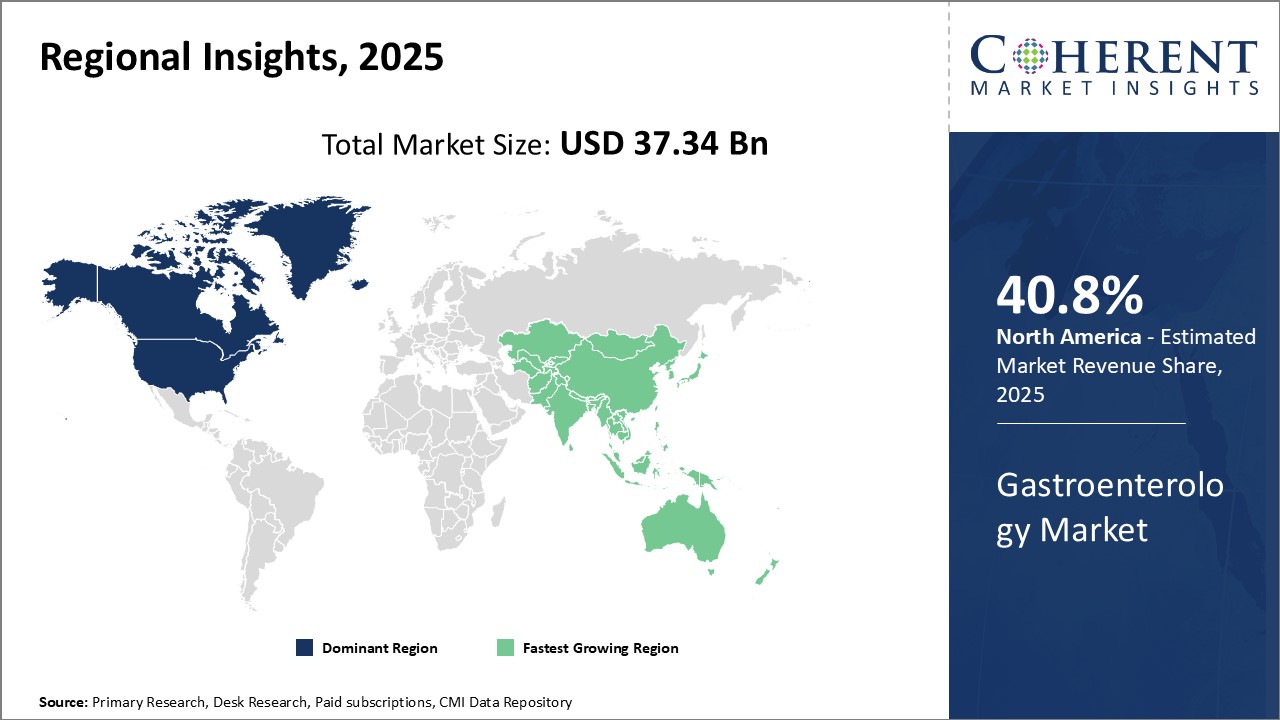
- By Region, North America hold the largest market share of 40.8% in 2025 as Adoption of Minimally Invasive Procedures.
To learn more about this report, Download Free Sample
Market Overview
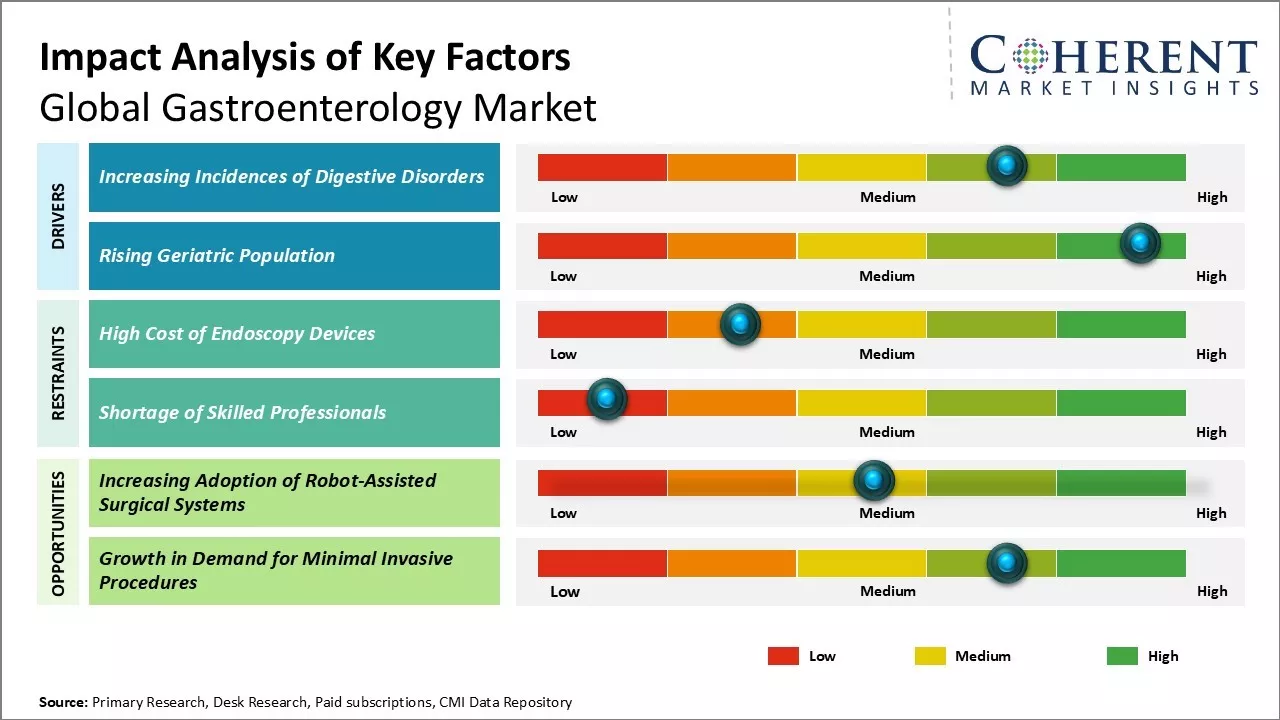
Global gastroenterology market growth is driven by the rising demand for minimally invasive procedures. Technological advancements in endoscopy such as high-definition imaging, narrow band imaging, capsule endoscopy, and others boosts the preference for endoscopy among patients and healthcare providers alike. Furthermore, increasing cases of gastrointestinal diseases due to change in lifestyle and eating habits has increased the need for early diagnosis and treatment. Other factors influencing the market growth include increasing healthcare expenditures in emerging economies and rising number of gastrointestinal disorder patients. Growing geriatric population also boosts demand. However, stringent regulatory approvals and high costs of gastrointestinal devices can hamper the market growth during the forecast period.
Current Events and their Impact on the Gastroenterology Market
|
Current Events |
Description and its impact |
|
Technological Advancements in AI and Endoscopy |
|
|
Supply Chain Challenges and Trade Tariffs |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Role of AI (Artificial Intelligence) in the Gastroenterology Market
AI is transforming the gastroenterology market by enhancing diagnostic accuracy and enabling early disease detection. Advanced algorithms help identify conditions like colorectal cancer, inflammatory bowel disease, and polyps more precisely than traditional methods. This technology streamlines workflow by automating data analysis, reducing the burden on specialists and speeding up diagnosis. In May 2025, Paige launched the Paige GI Suite, an AI-powered diagnostic tool that helps pathologists detect common gastrointestinal conditions. Unlike other AI solutions targeting a single tissue type, it supports AI analysis of biopsies from all GI tract regions, including the esophagus, stomach, duodenum, and colorectum.
Reimbursement Policies in the Gastroenterology Market
In the gastrointestinal industry, evolving reimbursement practices are reshaping the landscape for both patients and providers. The Centers for Medicare & Medicaid Services (CMS) proposed a 2.83% reduction in Medicare physician reimbursement for 2025, following a 1.25% cut in 2024. These continuous cuts raise significant concerns among healthcare professionals, particularly in rural and underserved areas where access to care remains limited. To address the need for streamlined quality reporting, CMS will implement the Gastroenterology Merit-based Incentive Payment System (MIPS) Value Pathway (MVP) in 2025, aligning specialty-specific practices with improved quality metrics and simplified reporting requirements.
Gastroenterology Market Insights, by Drug Type: Biologics/Biosimilar contribute the highest share of the market owing to Patent Expirations and Market Entry Opportunities.
Biologics and biosimilar medications have revolutionized the treatment of many gastrointestinal illnesses by directly targeting specific inflammatory mediators. These therapies disrupt particular pathways that trigger cytokine release or modulate immune responses with precision. As a result, biologics relieve symptoms more effectively and with fewer side effects than traditional medications. Anti-TNF drugs, for example, neutralize excess tumor necrosis factor-α—a key driver of gut inflammation—and have shown exceptional success in treating inflammatory bowel diseases. Physicians now prescribe biologics as first-line treatments for many chronic GI conditions because they address root causes rather than just symptoms. For Instance, the FDA has approved both subcutaneous and IV formulations of Otulfi, a Stelara biosimilar, for treating Crohn’s disease, ulcerative colitis, and all other indications approved for the original drug. This is further adding to the Gastroenterology Market share.
Gastroenterology Market Insights, by Dosage Form: Oral contributes the highest share of the market owing to Demand for Personalized Medicine.
Swallowing pills is generally more acceptable and less invasive than injections for long-term treatments. This makes the oral route particularly suitable for medications intended to control chronic diseases requiring lifelong medication usage. The ease of oral self-administration at home also facilitates adherence to prescribed dosing regimens. Many GI drug makers prioritize oral formulations to maximize user friendliness. As new entities are developed, oral dosage forms continue to be preferred whenever comparable efficacy to other options can be achieved. Such formulation advantages have positioned oral drugs as the mainstay of therapy for common gastrointestinal disorders. For Instance, in June 2024, ZUNVEYL's Dual MOA, the second oral therapy approved this decade, was designed to eliminate drug absorption in the gastrointestinal (GI) tract. It offers a long-term efficacy profile and aims to address some tolerability issues associated with leading Alzheimer's disease (AD) medications.
Gastroenterology Market Insights, by Disease TypeL: Inflammatory bowel diseases (IBD) contribute the highest share of the market owing to Increased Awareness and Early Diagnosis.
Crohn's disease and ulcerative colitis are chronic relapsing inflammatory disorders of unknown etiology that predominantly affect the bowel. Recent epidemiological studies indicate growing IBD incidence across both developed and developing regions globally as populations adopt western lifestyles. While genetic factors play a role, experts believe environmental triggers like gut microbiota imbalances and dietary changes can increase IBD burden. These long-lasting conditions demand long-term management with medication. Continued discovery of IBD pathophysiology has enabled improved drug development to reduce symptoms, induce remission, and prevent complications. As one of the most common gastrointestinal ailments, IBD represents a major focus area for gastroenterology therapeutics development. For Instance, in January 2025, Lilly announced that the FDA has approved Omvoh for the treatment of moderately to severely active Crohn's disease, making it the second interleukin-23p19 specific inhibitor authorized for both major forms of inflammatory bowel disease.
Gastroenterology Market Insights, by Distribution Channel, Hospital Pharmacies contribute the highest share of the market owing to Focus on Personalized Medicine.
Hospital pharmacists play a vital role in delivering cutting-edge biologic and biosimilar treatments that increasingly treat complex gastrointestinal disorders. They manage the specialized handling, storage, and administration these therapies require, which typically occur in hospital settings. They also leverage advanced diagnostic technologies, such as high-definition endoscopy and capsule endoscopy, to enhance the accuracy of diagnosing and planning treatment for gastrointestinal conditions. By facilitating early detection and timely intervention, hospital pharmacists improve patient outcomes and elevate the overall standard of GI care. This is further escalating the Gastroenterology Market revenue.
Regional Insights
To learn more about this report, Download Free Sample
North America Gastroenterology Market Trends
Significant healthcare investments, an aging population, and a high frequency of gastrointestinal illnesses are driving the rapid expansion of the gastroenterology market in North America. The region improves patient outcomes and detection accuracy by adopting cutting-edge technologies such as AI-assisted endoscopy and capsule diagnostics early. Prominent firms like Medtronic and Boston Scientific diversify their product lines with advanced medical devices and biologic treatments to meet growing market demands. For Instance, in May 2025, RedHill Biopharma Ltd. (Nasdaq: RDHL) ("RedHill" or the "Company") today announced that it has supported an independent medical education grant to create a new two-part H. Pylori CME program by Medscape, aiming to enhance clinical knowledge and improve patient outcomes.
Asia Pacific Gastroenterology Market Trends
An aging population, rising gastrointestinal disease prevalence, and increasing healthcare spending drive the strong growth of the Asia Pacific gastroenterology market. Innovations in technology, such as AI-assisted endoscopy and capsule diagnostics, enhance treatment outcomes and early detection. Improved healthcare systems and growing awareness of gastrointestinal health fuel notable market growth in countries like China and India. Additionally, the development of novel biologic medicines and the adoption of minimally invasive techniques contribute to expanding the market. For Instance, In September 2024, Lupin stated that it has signed a non-exclusive patent license agreement with Takeda Pharmaceutical Company (Takeda) to market Vonoprazan Tablets in India. Vonoprazan, a new potassium-competitive acid blocker (P-CAB), offers unique qualities that set it apart from other conventional acid suppressants like PPIs. These include meal-independent dosing, a longer duration of action that effectively controls nocturnal acid breakthrough, and full proton pump inhibition with the first dose.
United States Gastroenterology Market Trends
Factors such as an aging population, rising gastrointestinal problems, and advancements in medical technology drive significant growth in the United States gastroenterology market. Technological advancements like high-definition imaging and AI-assisted diagnostics improve early disease identification and treatment outcomes. Additionally, the growing use of biologics and biosimilars transforms the therapeutic landscape by offering tailored treatments for diseases such as Crohn's disease and inflammatory bowel disease. Together, these elements actively propel the dynamic evolution of the gastroenterology market in the United States. For Instance, In May 2023, Kohlberg & Company, LLC ("Kohlberg"), a renowned private equity firm with over 30 years of experience based in New York, announced that it has made a growth capital investment in United Digestive (the "Company"), a leading gastroenterology platform offering gastrointestinal ("GI") services throughout the Southeast United States.
India Gastroenterology Market Trends
Aging populations, rising rates of gastrointestinal illnesses, and improvements in medical technology are driving significant growth in the gastroenterology market in India. Technological advancements such as AI-assisted endoscopy and capsule diagnostics are improving early disease detection and treatment outcomes. Additionally, the growing use of biologics and biosimilars is transforming the therapeutic landscape by offering tailored treatments for diseases like Crohn's disease and inflammatory bowel disease. Together, these factors actively support the dynamic development of India’s gastroenterology market. For Instance, In May 2025, Abbott India announced that it and Takeda Pharmaceuticals Company have signed a non-exclusive patent license deal to commercialize Vonoprazan, a new chemical in gastrointestinal medicine, under the Vonefi brand.
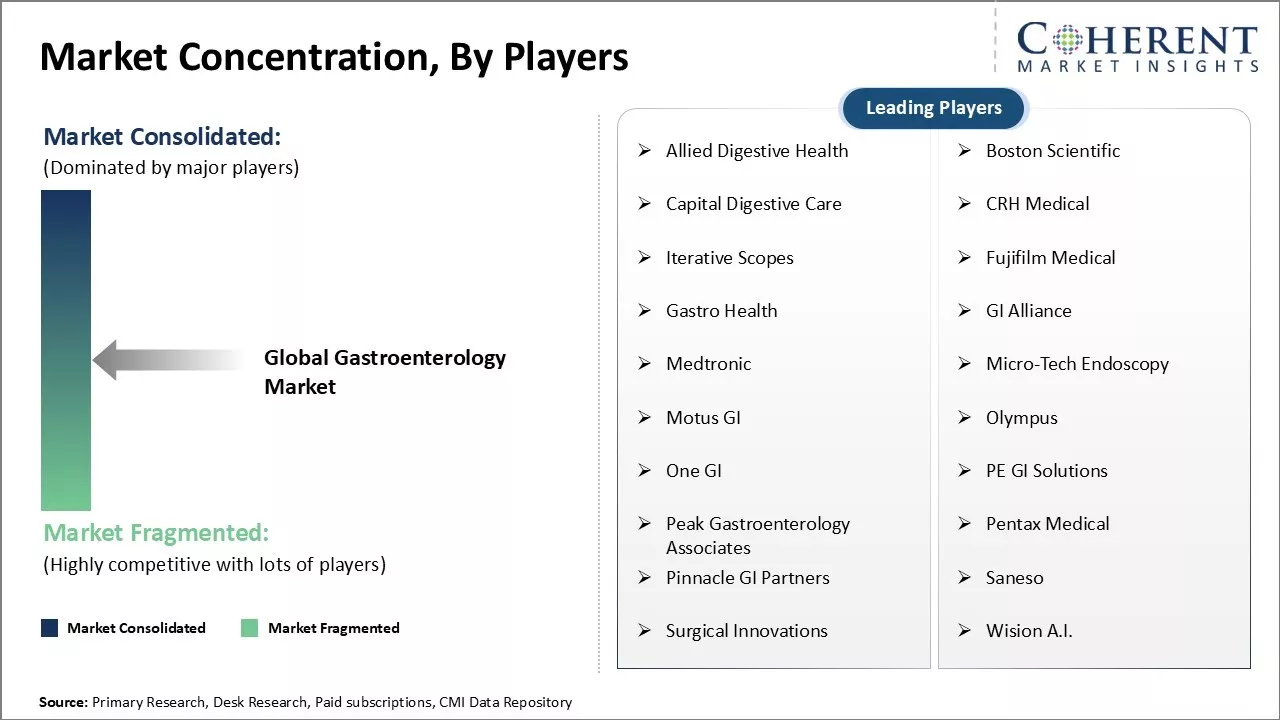
Market Concentration and Competitive Landscape
To learn more about this report, Download Free Sample
Gastroenterology Market News
- In April 2025, Kolkata's Desun Hospital and Agartala's Medicaid Pathological Lab and X-Ray Clinic launched a state-of-the-art gastroenterology and Cancer Clinic.
- In November 2024, Washington Gastroenterology (WaGI) partnered with WovenX Health to utilize its telehealth platform, aiming to improve GI care and patient outcomes.
- In August 2024, Indian gastroenterologists launched the Gastro AI Academy to offer free AI education to GI physicians and surgeons, enhancing their tech skills for better patient care. FUJIFILM India joined as an academic partner.
- In July 2024, Zydus Lifesciences, a multinational life sciences company, has signed a licensing agreement with Takeda Pharmaceutical Company to introduce the gastrointestinal medication Vault (Vonoprazan) to the Indian market. The company will use Vault, a potassium-competitive acid blocker (P-CAB), to treat GERD and other acid-related conditions.
Market Report Scope
Gastroenterology Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 37.34 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 6.3% | 2032 Value Projection: | USD 57.30 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Allied Digestive Health, Boston Scientific, Capital Digestive Care, CRH Medical, Iterative Scopes, Fujifilm Medical, Gastro Health, GI Alliance, Medtronic, Micro-Tech Endoscopy, Motus GI, Olympus, One GI, PE GI Solutions, Peak Gastroenterology Associates, Pentax Medical, Pinnacle GI Partners, Saneso, Surgical Innovations, Wision A.I. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Gastroenterology Market Trends
Increasing incidences of digestive disorders
Global gastroenterology market growth is driven by rising incidences of various digestive disorders across both developed and developing regions of the world. Digestive disorders and conditions such as gastroesophageal reflux disease (GERD), peptic ulcers, inflammatory bowel disease (IBD), irritable bowel syndrome (IBS), and liver diseases have become highly prevalent globally, thus, affecting people of all age groups. High consumption of junk food rich in fats and oils, increasing stress levels, unhealthy lifestyles, pollution, and consumption of alcohol and tobacco has significantly contributed to rising cases of indigestion, acidity, ulcers and other stomach issues. Moreover, genetic predisposition and changing dietary habits has led to increase in incidences of IBD conditions like ulcerative colitis and Crohn's disease especially in developed nations of North America, Western Europe and other developed Asian economies.
Rising geriatric population
Aging is usually accompanied by declining organ functions and rising vulnerability of various age-related diseases. Digestive disorders are highly common among the elderly population due to a number of physiological changes that naturally occur with aging. Factors like reduced stomach acid secretion, reduced digestion and absorption functions of the gastrointestinal tract, reduced gastric motility and delay in bowel movement predispose the elderly to gastrointestinal problems. Common age-related GI issues include gastroesophageal reflux, constipation, incontinence and risks of colon cancer.
Gastroenterology Market Opportunity
Increasing adoption of robot-assisted surgical systems
Global gastroenterology market can witness growth opportunities due to increasing adoption of robot-assisted surgical systems. Robotics offers several advantages over conventional laparoscopic surgeries such as high dexterity, 3D visualisation, tremor filtering and motion scaling. These features allow for improved surgical precision, flexibility and control, which enables better clinical outcomes. Gastroenterologists uses robotic platforms for conducting complex procedures such as bariatric surgeries, colorectal surgeries and Heller's myotomy. This boosts demand for surgical robots and associated consumables. Furthermore, robotic systems have potential to make minimally invasive surgeries accessible to sites without trained laparoscopic surgeons, which can expand the reach of quality care. With ongoing technological advancements, robotics can witness greater acceptance in gastrointestinal surgery over the forecast period. This presents lucrative growth opportunities for players operating in the surgical robotics segment.
Analyst Opinion (Expert Opinion)
- Innovation-driven precision medicine and digital integration will determine the winners and losers as the gastroenterology market enters a revolutionary phase. I believe rising cases of complex chronic GI disorders like Crohn’s and ulcerative colitis require a drastic shift from symptomatic treatment to targeted biologic medicines. Biologics such as anti-TNF drugs have demonstrated remission rates up to 60% in moderate to severe cases, outperforming traditional treatments.
- Integrating AI-powered diagnostics into endoscopy has become a necessity rather than a luxury. Studies show that AI-assisted colonoscopy increases polyp detection rates by 15% to 20%, directly enabling earlier colorectal cancer detection. Companies like Medtronic, with their GI Genius system, set new benchmarks for clinical efficacy. Players who fail to adopt these technologies risk rapid obsolescence.
- Addressing the stark disparities in pricing and accessibility, especially in emerging markets, remains critical for market survival. Although biologics improve clinical outcomes, their high costs limit widespread use. Biosimilars offer up to 30% cost savings, as real-world evidence from the EU shows, but physician hesitation and regulatory barriers slow their adoption. Market leaders will drive accessibility through strategic partnerships and patient-centric pricing alongside therapeutic innovation.
- In conclusion, gastroenterology is rapidly evolving into a precision-driven, technology-enabled specialty where clinical outcomes, patient satisfaction, and cost-effectiveness must align seamlessly for sustained success. The field no longer focuses solely on symptom management.
Market Segmentation
- By Drug Type Insights (Revenue, USD Bn, 2020 - 2032)
- Biologics/Biosimilars
- Antacids
- Laxatives
- Antidiarrheal Agents
- Antiemetics
- Antiulcer Agents
- Others
- By Dosage Form Insights (Revenue, USD Bn, 2020 - 2032)
- Oral
- Parenteral
- Others
- By Disease Type Insights (Revenue, USD Bn, 2020 - 2032)
- Inflammatory Bowel Diseases (IBD)
- Crohn's Disease
- Ulcerative Colitis
- Irritable Bowel Syndrome (IBS)
- Gastroesophageal Reflux Disease (GERD)
- Liver Diseases
- Colorectal Cancer
- Others (Peptic Ulcers, etc.)
- By Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacies
- Retail Pharmacies
- Online Pharmacies
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Player Insights
- Allied Digestive Health
- Boston Scientific
- Capital Digestive Care
- CRH Medical
- Iterative Scopes
- Fujifilm Medical
- Gastro Health
- GI Alliance
- Medtronic
- Micro-Tech Endoscopy
- Motus GI
- Olympus
- One GI
- PE GI Solutions
- Peak Gastroenterology Associates
- Pentax Medical
- Pinnacle GI Partners
- Saneso
- Surgical Innovations
- Wision A.I
Sources
Primary Research interviews:
- Interviews with gastroenterologists
- Discussions with hospital pharmacists specializing in GI treatments
- Conversations with medical device manufacturers’ R&D teams
Databases:
- PubMed
- ClinicalTrials.gov
- WHO Global Health Observatory
Magazines:
- Gastroenterology & Endoscopy News
- Medical Device News Magazine
- Pharma Manufacturing Magazine
Journals:
- The American Journal of Gastroenterology
- Gut (British Society of Gastroenterology)
- Gastroenterology (Journal of the American Gastroenterological Association)
Newspapers:
- The New York Times Health Section
- The Guardian Health News
- The Washington Post Health & Science
Associations:
- American Gastroenterological Association (AGA)
- European Crohn’s and Colitis Organisation (ECCO)
- International Society for Gastrointestinal Hereditary Tumors (InSiGHT)
Public Domain sources:
- U.S. Food and Drug Administration (FDA) databases
- Centers for Disease Control and Prevention (CDC) reports
- World Health Organization (WHO) publications
Proprietary Elements:
- CMI Data Analytics Tool, and Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients