Fill Finish Manufacturing Market Size and Trends
The fill finish manufacturing market is estimated to be valued at USD 17.95 Bn in 2025 and is expected to reach USD 33.69 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 9.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
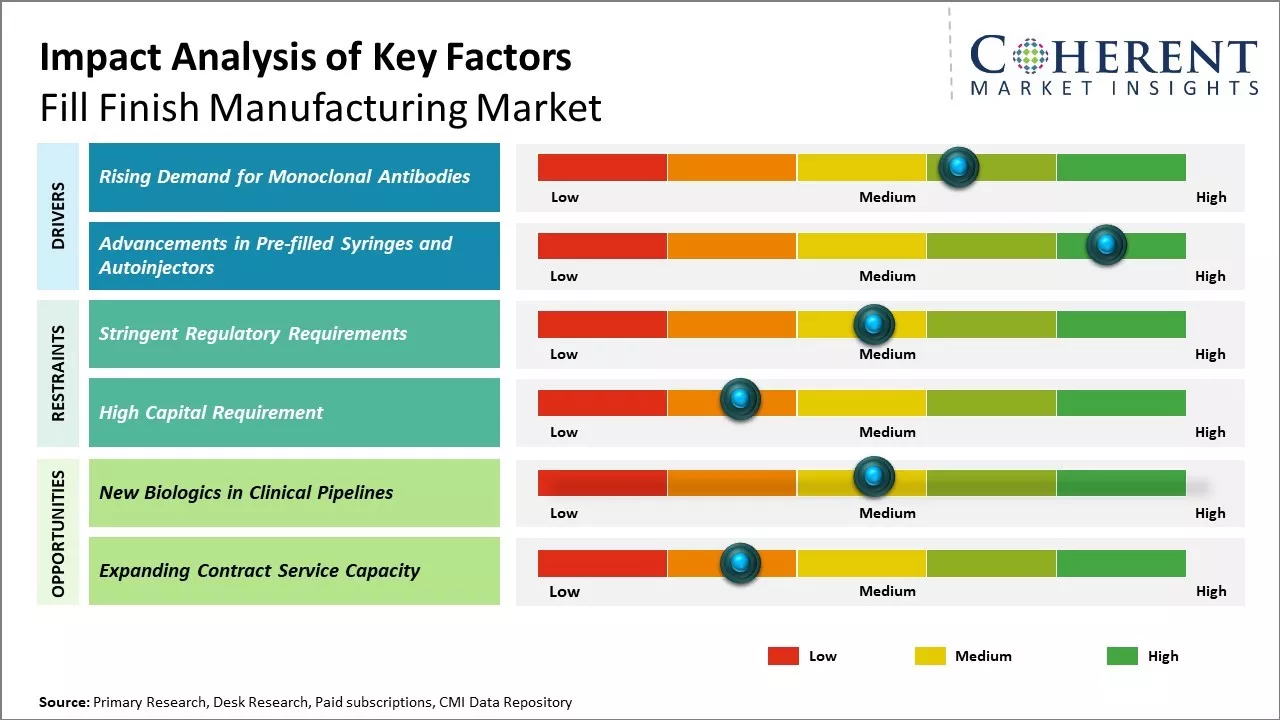
The fill finish manufacturing market is witnessing strong growth driven by the robust expansion of the global biologics industry. The demand for biologics is increasing rapidly due to their higher specificity and efficiency over traditional drugs. Moreover, the increasing demand for vaccines and monoclonal antibodies is also supporting the growth of the fill finish manufacturing stage. However, the market faces some challenges in the form of high capital investment requirements for facilities and equipment as well as stringent regulatory norms regarding sterile manufacturing of biologics drugs.
Market Driver – Rising Demand for Monoclonal Antibodies
The production of monoclonal antibodies has seen tremendous growth in recent years owing to increased research and development into targeted cancer immunotherapies and new biologic drug formulations. Monoclonal antibodies have emerged as the largest and fastest growing segment of the biologics industry due to their ability to treat a wide range of medical conditions with high specificity. Leading drug makers are aggressively pursuing monoclonal antibody candidates for diseases such as rheumatoid arthritis, psoriasis, multiple sclerosis, and various cancers. As the monoclonal antibody landscape expands with more approvals and late-stage candidates, biologics manufacturers are struggling to keep pace with the rising demand. This has fueled significant capital expenditure towards expanding fill finish production capacity that can efficiently and precisely handle the unique challenges of monoclonal antibody drug substances. Top players in the biologics drug market have prioritized long-term deals with fill finish providers that can guarantee supply over the next five to ten years as the monoclonal antibody market is expected to grow exponentially.
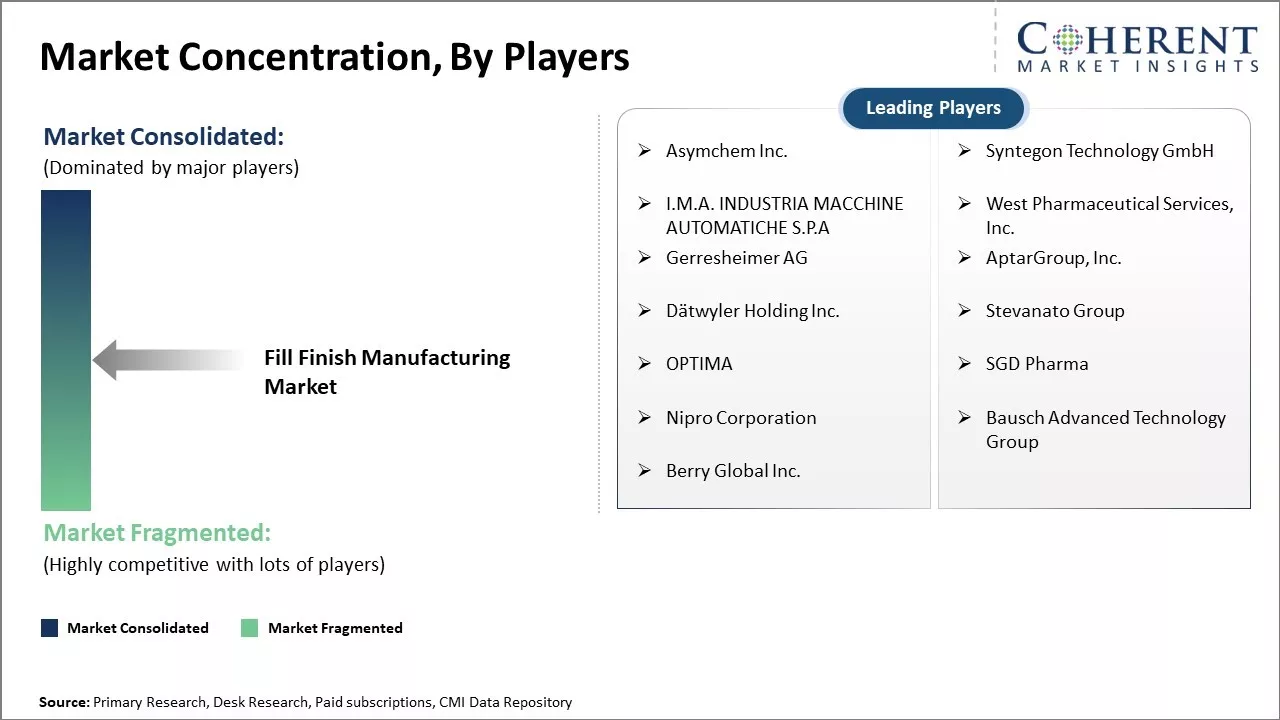
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Market Driver – Advancements in Pre-filled Syringes and AutoinjectorsA growing preference among patients and care providers for drug delivery systems like pre-filled syringes and autoinjectors has biomedicine companies aggressively pursuing novel formulation strategies. Self-administration options improve compliance for chronic disease therapy and also enable subcutaneous delivery of biologics that were previously only available via intravenous methods. The development of highly concentrated biologic dosage forms has enabled devices like pre-filled syringes and autoinjectors that lower injection volumes and frequencies. However, working with such formulation poses several manufacturing complexities including ensuring drug substance stability in a small deliverable volume. Biologics fill finish providers are making extensive capital investments in automation technologies, isolator systems, and lyophilization capabilities to address these complexities. Leading contract manufacturing organizations are also developing specialized expertise in assembly, packaging and labeling of combination products. This is helping biopharmaceutical innovators to increasingly adopt advanced drug delivery systems and expand patient access to self-administered biologic therapies.

To learn more about this report, Download Free Sample
Market Challenges – High Capital RequirementThe high capital requirement poses a major challenge for the growth of the fill finish manufacturing market. Setting up facilities for biologics manufacturing is an extremely capital-intensive process which requires investments running into hundreds of millions of dollars. This is because biologics manufacturing facilities need to meet stringent regulatory standards and require advanced technologies and critical, specialized equipment to facilitate aseptic fill finish processes. The costs associated with ensuring required cleanroom specifications, prevention of contamination, and validation of equipment is very high.
Market Opportunities – Expanding Contract Service Capacity
Expanding contract service capacity in biologics fill finishing can tap into significant opportunities in the global market. As biopharmaceutical development continues to grow in complexity, an increasing number of drug manufacturers are outsourcing non-core functions to contract service providers. Fill finishing, being a highly technical late-stage process in biologics production, is well-suited for outsourcing.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
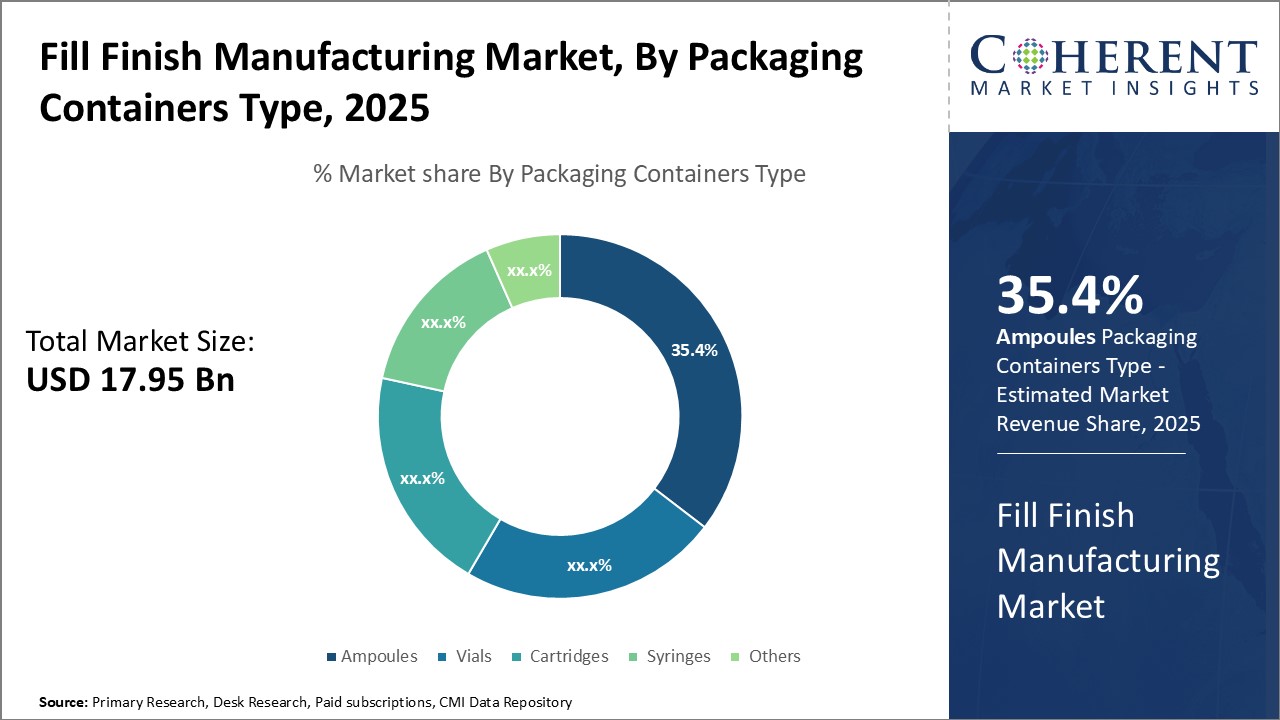
Insights, By Packaging Containers Type: Growing Preference for Single Dose Packaging Drives Ampoules SegmentThe packaging containers type segment includes ampoules, vials, cartridges, syringes, and others. The ampoules segment is estimated to hold 35.4% share of the market in 2025 owing to its advantages as a single dose packaging format. Ampoules offer benefits such as preventing bacterial contamination between doses, ease of transport as they do not require additional packaging, and consistency in dose volume and delivery. These features make ampoules particularly suited for treatments that require precise dosing. A key factor driving increased adoption of ampoules is the growth of therapies targeting niche patient populations or specialized indications. Developing drugs for rare diseases or personalized medicine often involves small clinical trials with a limited number of patients. Ampoules allow for minimizing waste from unused portions of multi-dose vials in these situations. They also eliminate the risk of dose measurement errors and ensure each patient receives the exact amount prescribed by their physician. Overall, the growing need for safe, reliable dosing of novel and specialized therapies is a primary driver propelling the ampoule segment. Ampoules meet the stringent dosing and delivery demands of personalized medicines in development pipelines and clinical trials. Their single use format and robust stability also provide advantages over multi-dose packaging when distributing treatments targeting rare or small patient populations.
Insights, By Biologics Type: Cardiac Care through Imaging
The biologics type segment includes antibodies, cell therapies, oligonucleotides, recombinant proteins, vaccines, and others. The antibodies segment is estimated to account for 32.3% of the market share in 2025, owing to their widespread successful commercial applications across therapeutic areas. Monoclonal antibodies have revolutionized treatment across various disease classes like cancer, autoimmune disorders, and infectious diseases due to their high target specificity. A key factor underpinning antibody growth is their versatility. Monoclonal antibodies can be engineered to serve as monotherapies or play supporting roles in combination regimens. Their mechanisms of action also demonstrate diverse modalities like blocking receptor signaling, recruiting immune cells to enhance responses, or delivering drugs directly to tumors. Such diversity enables antibodies to impact multiple disease pathways. Additionally, advancements in antibody engineering now allow tailored formats like bispecific, antibody drug conjugates, and next-generation Fc regions with improved properties. These newer modalities utilize antibodies as targeting vectors to expand the modality’s therapeutic potential. Such innovation extends antibodies’ commercial lives and maintains interest in their development.
Insights, By End User: CMOs Lead as Biopharma Outsource Complex Manufacturing
The end user segment includes contract manufacturing organization, biopharmaceutical companies, and others. The contract manufacturing organization segment is estimated to hold 39.8% of the market share in 2025 as biopharmaceutical companies increasingly outsource fill finish operations. Developing and manufacturing biologics internally requires significant capital investment and specialized expertise that many biopharma lack, particularly smaller firms. Rather than build expensive, complex manufacturing facilities in-house, biopharmaceutical companies now commonly engage Contract Manufacturing Organizations to handle fill finish and related downstream activities. CMOs allow biopharma to focus resources on drug development while mitigating investment risk from manufacturing.
Regional Insights
Need a Different Region or Segment? Download Free Sample
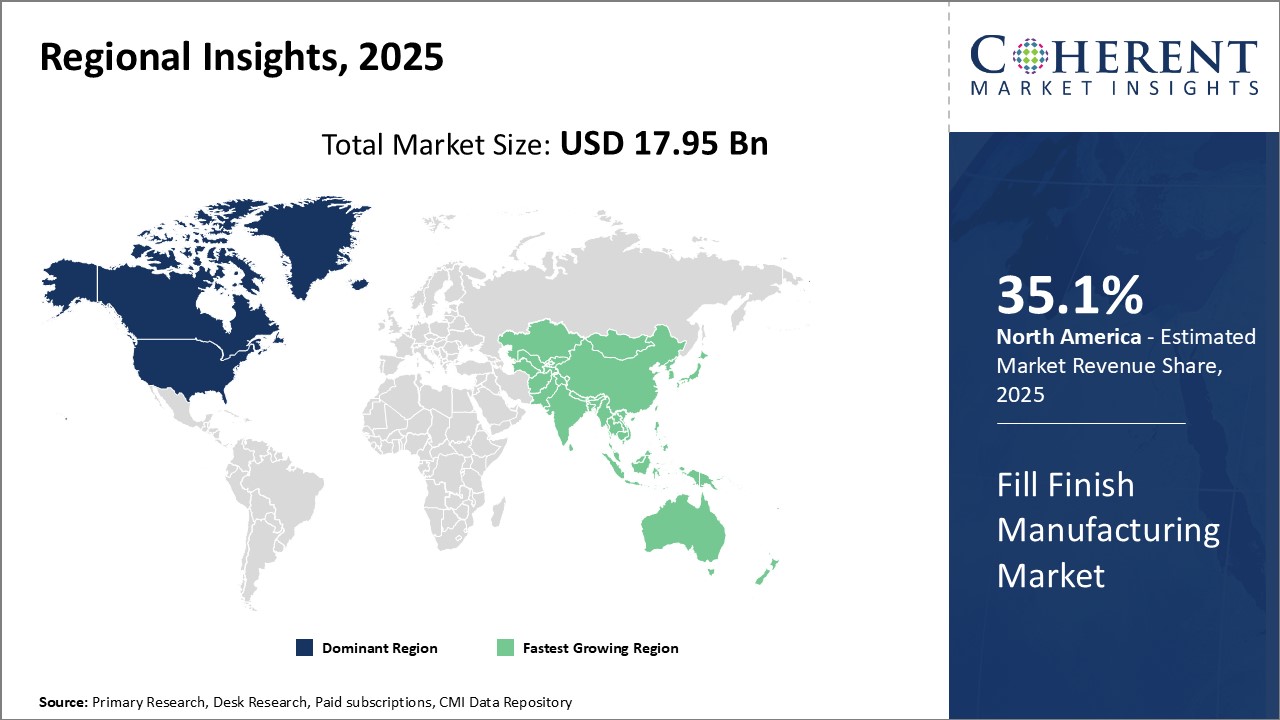
North America remains the dominant region in the global fill finishing manufacturing market and is estimated to hold 35.1% of the market share in 2025. North America has dominated the global fill finish manufacturing market for the past decade owing to strong industry presence and strategic expansions by leading players. Major pharmaceutical companies in the U.S. have their biologics development and large-scale manufacturing facilities located in-house or through contract service providers. This allows them to gain control over the entire supply chain and ensure timely availability of approved biologics to patients.
However, their dominance is now being challenged by growing markets in Asia Pacific region, especially China. The country has emerged as the fastest growing regional market for biologics fill finish as local pharmaceutical firms adopt international quality standards to meet rising domestic and export demand. Several Chinese companies are partnering with large multinational contract service providers to set up state-of-the-art fill finish facilities with the latest technology and skilled workforce. Moreover, favorable government policies like tax exemptions on imports of necessary equipment and lower operating costs compared to developed markets are also attracting investments.
Market Report Scope
Fill Finish Manufacturing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 17.95 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.4% | 2032 Value Projection: | USD 33.69 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Asymchem Inc., Syntegon Technology GmbH, I.M.A. INDUSTRIA MACCHINE AUTOMATICHE S.P.A, West Pharmaceutical Services, Inc., Gerresheimer AG, AptarGroup, Inc., Dätwyler Holding Inc., Stevanato Group, OPTIMA, SGD Pharma, Nipro Corporation, Bausch Advanced Technology Group, and Berry Global Inc. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Fill Finish Manufacturing Industry News
- On February 8, 2024, Alcami Corporation, a contract development and manufacturing organization (CDMO), announced the strategic expansion of its sterile fill/finish manufacturing capacity with the addition of a new sterile fill/finish line with isolator and two lyophilizers at its existing Charleston, SC manufacturing campus
- On January 24, 2024, Stevanato Group, a global provider of drug containment and delivery solutions to the pharmaceutical, biotechnology, and life sciences industries, announced that it had introduced two new offerings for efficient small batch pharmaceutical manufacturing: the EZ-fill Kit and the non-GMP laboratory fill and finish service at its Technology Excellence Centers (TEC). Stevanato Group’s EZ-fill Kit builds on its existing ready-to-use platform of pre-sterilized containment solutions – vials, cartridges, and syringes – allowing customers to effectively screen different primary packaging in combination with drug products
- In September 2023, Syntegon Technology GmbH, a global process & packaging technology provider, announced that it had showcased new developments for the filling and processing of small and micro batches At CPHI Barcelona 2023, Spain held from October 24-26, 2023. Equipment designed specifically for small batch sizes is in high demand not only for research and development, but also for the commercial production of liquid and solid pharmaceuticals.
*Definition: The fill finish manufacturing involves the sterile filling and packaging of biologic therapies such as monoclonal antibodies, recombinant proteins, vaccines, and other injectable therapeutics. This final stage in biologic drug production requires specialized aseptic processing equipment and techniques to fill vials, syringes and other containers while maintaining product and personnel sterility.
Market Segmentation
- Packaging Containers Type Insights (Revenue, USD Bn, 2020 - 2032)
- Ampoules
- Vials
- Cartridges
- Syringes
- Others
- Biologics Type Insights (Revenue, USD Bn, 2020 - 2032)
- Antibodies
- Cell Therapies
- Oligonucleotides
- Recombinant Proteins
- Vaccines
- Others
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Contract Manufacturing Organization
- Biopharmaceutical Companies
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Asymchem Inc.
- Syntegon Technology GmbH
- I.M.A. INDUSTRIA MACCHINE AUTOMATICHE S.P.A
- West Pharmaceutical Services, Inc.
- Gerresheimer AG
- AptarGroup, Inc.
- Dätwyler Holding Inc.
- Stevanato Group
- OPTIMA
- SGD Pharma
- Nipro Corporation
- Bausch Advanced Technology Group
- Berry Global Inc.
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
