Esoteric Testing Market Size and Forecast – 2025-2032
The Esoteric Testing Market is estimated to be valued at USD 32.09 Bn in 2025 and is expected to reach USD 67.89 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 11.3% from 2025 to 2032.
Key Takeaways
- Based on Type, the Molecular Diagnostics segment is expected to dominate the market during the forecast period, owing to the growing focus on personalized medicine.
- Based on Technology, Mass Spectrometry technologies will account for a significant market share in 2025, driven by their high accuracy, rapid results, and expanding applications in advanced diagnostic testing.
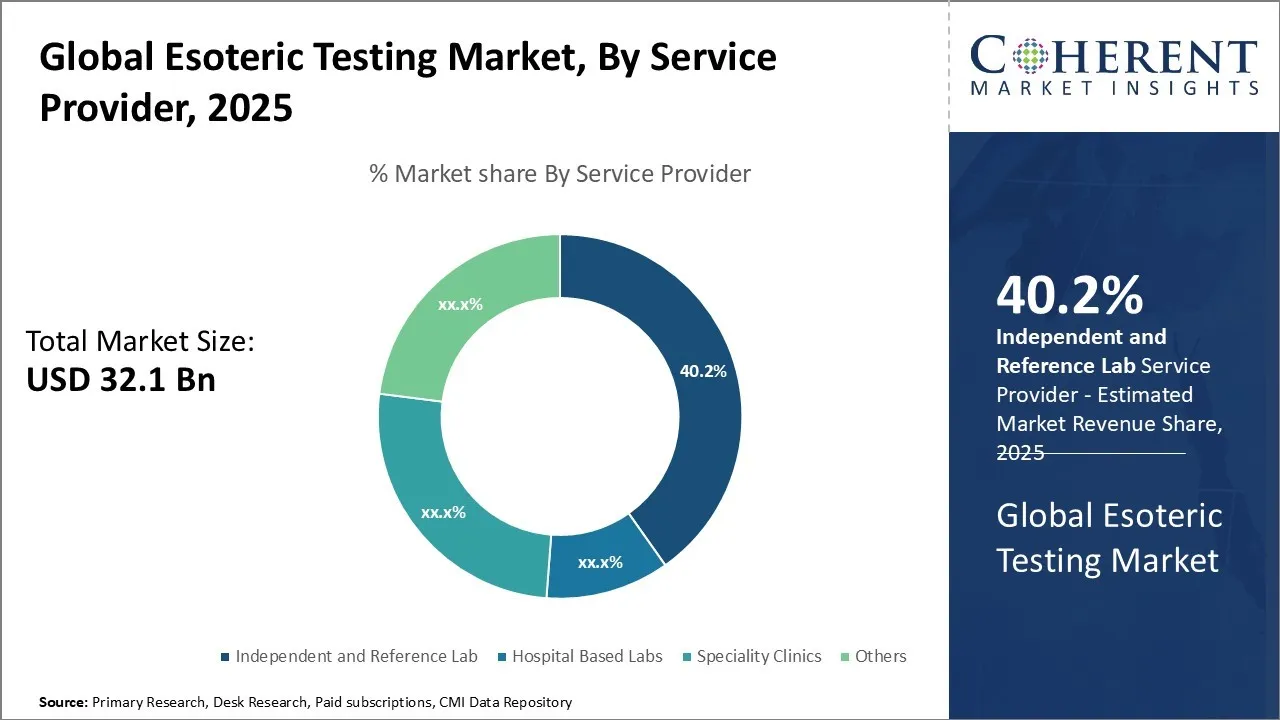
- Based on Service Provider, Independent And Reference Laboratories are projected to lead the market by 2025, benefiting from technological innovation, broad service offerings, and strategic partnerships with healthcare providers.
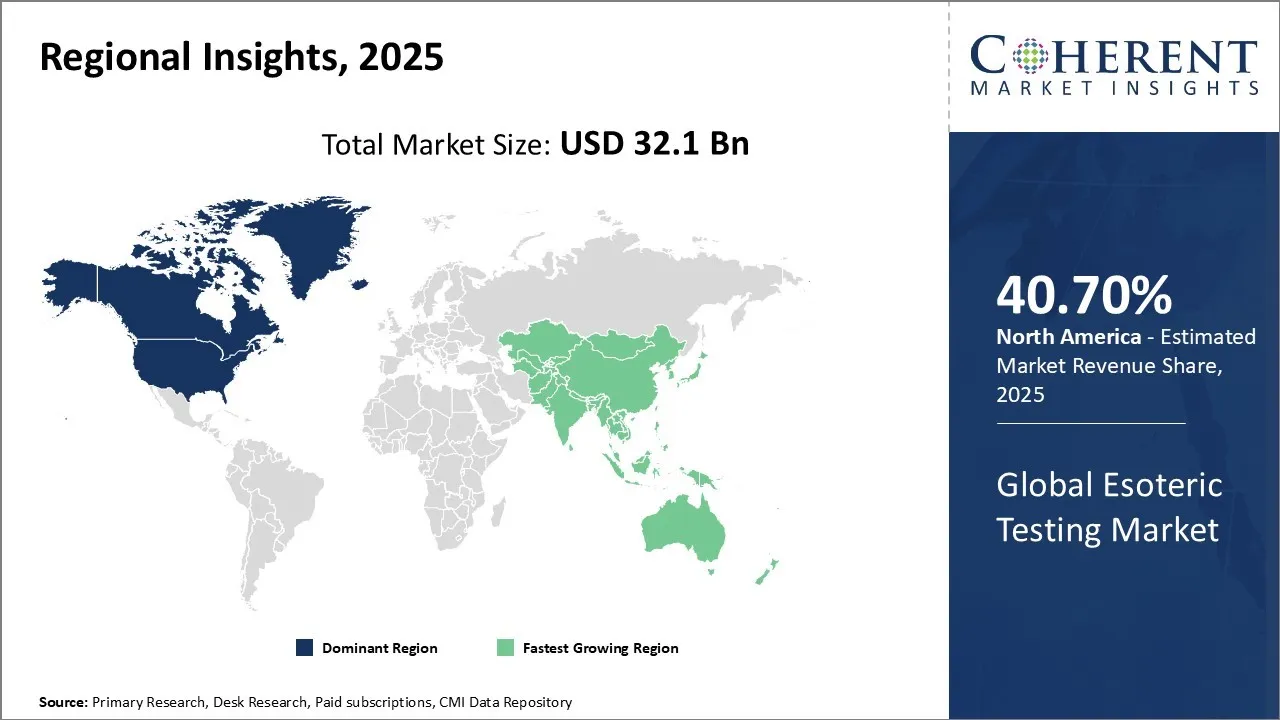
- Based on Region, North America will dominate the esoteric testing market with a 40.7% share in 2025 due to high disease burden and the strong presence of major diagnostic players.
Market Overview
The esoteric testing market is witnessing strong growth, driven by the growing need for specialized diagnostic tools. These tests, which examine DNA, RNA, proteins, metabolites, and antibodies, are crucial to understanding a person's genetic and molecular makeup. They are essential in Neurodiagnostic and treating chronic diseases like cancer and unusual infections. The growing burden of chronic diseases across the globe, rapid technological advances, and growing focus on individualized therapeutic approaches fuel the market. Favorable reimbursement terms and strategic partnerships between diagnostics and pharma players are also enhancing the availability and affordability of esoteric tests.
Current Events and Their Impact on Esoteric Testing Market
|
Current Events |
Description and its impact |
|
Technological Advancements in Diagnostic Methods |
|
|
Economic and Market Expansion Dynamics |
|
|
Public Health and Demographic Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Reimbursement Scenario in Esoteric Testing Market
CMS governs reimbursement policies for Medicare and Medicaid, covering tests deemed “medically necessary.” Medicare is particularly crucial as a significant payer in the U.S. healthcare system.
- Coverage for Esoteric Testing: Medicare reimburses certain esoteric tests, especially genetic tests for cancer patients, under specific conditions like hereditary cancer risk assessments. For example, patients with a family history of cancer might be eligible for testing that assesses genetic predispositions.
- Coverage Limitations: Despite this, CMS has strict guidelines. Tests like pre-symptomatic genetic assessments and tests for undiagnosed disease susceptibility are generally excluded from reimbursement. This creates challenges in situations where early detection could significantly impact patient care, such as in pre-symptomatic genetic conditions.
Esoteric Testing Market Insights, By Service Provider: Independent and Reference Labs lead with specialized capabilities and broad accessibility
Independent and reference laboratories are positioned to lead the service provider landscape by 2025, benefiting from their ability to offer high-complexity testing services, rapid turnaround times, and widespread sample collection networks. An April 2024 publication in the New England Journal of Medicine emphasized how independent labs like academic medical centers played a pivotal role in scaling up genomic testing services during emerging health crises, including outbreaks of antimicrobial resistance and new viral pathogens. Hospital-based labs and specialty clinics are also experiencing a rise in esoteric testing volumes, especially in neurology and oncology specialties, with decentralized access to molecular and immunological diagnostics. Specialty clinics are increasingly adopting esoteric testing to differentiate themselves with personalized diagnostic offerings, particularly in fields like fertility treatment and rare genetic disorders.
Esoteric Testing Market Insights, By Type- Molecular Diagnostics spearheads growth due to precision medicine advancements
The esoteric testing market on the basis of Type is being segregated into Endocrinology, Oncology, Molecular Diagnostics, Neurology, Immunology, Genetic Testing, and Others, where Molecular diagnostics will dominate the largest portion in the esoteric testing market due to increased demand for precision medicine, cancer genomics, and detection of infectious disease. Advances in technology such as next-generation sequencing (NGS) and CRISPR-based diagnostics are widening molecular diagnostic potential. For example, in January 2024, Nature Medicine reported on the use of CRISPR-Cas technology to create ultra-sensitive point-of-care diagnostics for infectious disease testing, including tuberculosis. Increased use of molecular diagnostics in individualized care is facilitated by the fact that it can deliver early, precise, and actionable data to treat patients. Likewise, esoteric oncology tests are becoming more prevalent with the widening of liquid biopsy technologies for non-invasive cancer detection.
Esoteric Testing Market Insights, By Technology: Real-time PCR and Mass Spectrometry dominate technological adoption
The mass spectrometry serves as fundamental tools in esoteric testing technology markets because these technologies allow quick disease detection with precise accuracy and adaptability. Clinical diagnostics evolve through mass spectrometry due to the fact that this technology permits accurate identification of intricate biomarkers and metabolic pathways. The University of California, UCSFS department, published in JAMA Network Open (March 2024) that proteomic profiling using mass spectrometry performed better than cholesterol testing for cardiovascular disease risk assessment. Although they play vital functions, immunology and oncology and infectious diseases diagnostics continue to be enriched by newer technologies which include flow cytometry and chemiluminescence and ELISA and radioimmunoassay as well as newer methods to the growing esoteric tests menu.
Regional Insights
To learn more about this report, Download Free Sample
North America Esoteric Testing Market Trends
North America is expected to be the largest market for esoteric testing during the forecast period, accounting for over 40.7% of the market share in 2025. The growth of the market in North America is attributed to the high incidence of target diseases, favorable reimbursements, and the presence of leading service providers in the region. Growth is primarily driven by the high prevalence of chronic diseases, favorable reimbursement structures, and the robust presence of advanced diagnostic laboratories. The Centers for Medicare & Medicaid Services (CMS) expanded coverage for next-generation sequencing (NGS) tests for inherited cancer syndromes in the U.S. in March 2023, boosting demand for molecular diagnostics. Also, in January 2025, Canada's Ontario Health agency announced the launch of an advanced genomics diagnostic program integrating whole-genome sequencing for rare diseases, further enhancing the esoteric testing market growth.
Latin America Esoteric Testing Market Trends
Latin America is emerging as a steadily growing region in the esoteric testing space, driven by improving healthcare access and growing investments in advanced diagnostics. In October 2024, Brazil’s Ministry of Health launched a national program to integrate genetic testing and rare disease diagnostics across public healthcare systems (SUS), significantly boosting demand for esoteric tests like genetic panels. The Mexican government, through IMSS, initiated funding for early cancer diagnosis programs in 2023, utilizing advanced biomarkers and molecular tests. These factors are further adding to the esoteric testing market share.
Europe Esoteric Testing Market Trends
Europe
is expected to be the second-largest market for esoteric testing, accounting for over28.3%
of the market share in 2025. The growth of the market in Europe is attributed to the availability of research funding and investments in the biotechnology sector in the region. Germany's Federal Ministry of Education and Research (BMBF) invested €50 million into the "Genome Germany" initiative to establish genomic medicine nationwide, announced in February 2024. The NHS Genomic Medicine Service expanded free cancer genomics testing in March 2024 to enhance early cancer diagnosis, integrating tests like NGS panels.Asia Pacific Esoteric Testing Market Trends
Asia Pacific
is expected to be the fastest-growing market for esoteric testing, growing with a CAGR of over14.2%
during the forecast period. The growth of the market in Asia Pacific is attributed to improving healthcare infrastructure, rising disposable incomes, and increasing awareness about advanced diagnostic tests in the region. In December 2024, China’s National Health Commission approved widespread integration of liquid biopsy tests into standard cancer diagnostic protocols, driving massive demand for esoteric diagnostics. Also, Indian Council of Medical Research (ICMR) initiated a major national genetic testing initiative for inherited disorders in April 2024, significantly boosting demand for molecular testing.Middle East Esoteric Testing Market Trends
The Middle East is gradually adopting esoteric testing, fuelled by growing precision medicine programs, especially in Israel and the Gulf countries. Israel’s Ministry of Health partnered with the Weizmann Institute in June 2024 to expand personalized genomics and rare disease diagnostics under the "National Genomics Plan." Abu Dhabi's Department of Health launched a precision medicine program in collaboration with G42 Healthcare to implement early disease detection via advanced molecular testing, reported in September 2024.
Esoteric Testing Market Drivers
Rising prevalence of chronic and infectious diseases
The rising prevalence of chronic diseases such as cancer, cardiovascular diseases, and neurological disorders is a major factor driving growth in the esoteric testing market. The global burden of chronic diseases is rapidly increasing. According to the World Health Organization, chronic diseases account for 71% of deaths worldwide. The early diagnosis of chronic diseases requires specialized esoteric tests such as genomic testing, molecular diagnostics, and oncology biomarker testing. These tests help in understanding disease prognosis, making treatment decisions and monitoring progression. The demand for esoteric tests is therefore rising for the diagnosis and management of chronic diseases.
Esoteric Testing Market Opportunities- Technological Innovations
Recent advances in genomics, proteomics, metabolomics, and molecular diagnostics have expanded the scope of esoteric testing. Technologies like next-generation sequencing allow high-speed sequencing of genes enabling genomic testing. Mass spectrometry and flow cytometry enable the identification of proteins and biomarkers from samples. Automation has increased the efficiency of esoteric labs. Labs are adopting advanced software solutions for managing sample tracking, analysis, and result reporting. Big data analytics enables insights from large volumes of patient data. The integration of advanced technologies is enhancing the capabilities of esoteric labs and driving market growth. For instance, on June 20, 2023, Diatech Pharmacogenetics, an Italy-based leader in the development, production, and commercialization of pharmacogenetics tests for cancer, established a collaboration agreement with Janssen Pharmaceuticals NV, a pharmaceutical company, with the aim of improving access to precision medicine for patients with bladder cancer.
Market Report Scope
Esoteric Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 32.09 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 11.3% | 2032 Value Projection: | USD 67.89 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
LabCorp, Quest Diagnostics, OPKO Health, Miraca Holdings, Myriad Genetics, Sonic Healthcare, Cardio Diagnostics Holdings, Inc, Veravas, BPGbio, Inc., Fulgent Genetics, Alveo Technologies, Inc., and S.R.L. Diagnostics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Recent Developments in Esoteric Testing Market
- In April 2024, Labcorp introduced a first-of-its-kind commercial blood test measuring glial fibrillary acidic protein (GFAP), a biomarker released into the bloodstream when astrocytes are damaged. This test aids in the early detection of neurodegenerative diseases and brain injuries, including Alzheimer's disease, multiple sclerosis, glioblastoma, and traumatic brain injury.
- In April 2024, Quest Diagnostics expanded its Alzheimer's disease diagnostic services by adding a blood test for phosphorylated tau 217 (p-tau217), a biomarker associated with the disease. This addition supports a comprehensive offering, including plasma biomarker testing alongside traditional cerebrospinal fluid and genetic tests.
- In March 2024, Biodesix announced a strategiccollaboration with Memorial Sloan Kettering Cancer Center. This partnership aims to develop advanced diagnostic tests to improve cancer treatment, focusing on creating esoteric tests that provide more precise and personalized cancer diagnostics.
- On June 21, 2023, Cardio Diagnostics Holdings, Inc, a pioneer in AI-driven precision cardiovascular medicine tests, announced the U.S. launch of PrecisionCHD. This groundbreaking test marks a significant milestone in the fight against coronary heart disease (CHD), the most common type of heart disease and the primary cause of heart attacks. It is the second product to be released from Cardio Diagnostics’ cutting-edge suite of AI-driven molecular cardiovascular disease technologies.
Analyst’s Opinion (Expert Opinion)
The esoteric testing market has become one of the most rapidly growing sectors in the larger healthcare diagnostics industry. A surge in investor interest is being witnessed, fuelled by the potential of the sector to transform diagnostic capability and meet sophisticated, unmet medical needs. Investors are increasingly investing in companies involved in advanced molecular diagnostics, genomics, proteomics, and next-generation sequencing (NGS), aware that innovation in esoteric testing can provide both high returns and high clinical impact. This investment influx is stimulating fast-paced technological innovations, such as the coupling of artificial intelligence (AI) with diagnostic testing, new biomarker discovery, and increased automation in sample analysis. Accordingly, esoteric testing is moving away from traditional single-analyte assays towards multi-omics approaches that allow for earlier and more precise diagnosis of conditions such as cancer, rare genetic disorders, autoimmune disorders, and complex infectious diseases. Moreover, the application of esoteric tests in individualized medicine is also raising their significance. Individualized diagnosis according to someone's own unique genetic or molecular profile is becoming more possible, allowing for targeted therapies that enhance patient response and minimize trial-and-error therapy and healthcare costs. For example, the emergence of precision oncology diagnostics to inform treatment decisions illustrates the move toward a more individualized, efficient model of care.
Market Segmentation
- By Type
- Endocrinology
- Oncology
- Molecular Diagnostics
- Neurology
- Immunology
- Genetic Testing
- Others
- By Technology
- Mass Spectrometry
- Real Time PCR
- Flow Cytometry
- Chemiluminescence
- ELISA
- Radioimmunoassay
- Others
- By Service Provider
- Independent & Reference Labs
- Hospital-based Labs
- Specialty Clinics
- Others
- By Region:
- North America
- Europe
- Asia Pacific
- Latin America
- Middle East
- Africa
- Top Companies in Esoteric Testing Market
- LabCorp
- Quest Diagnostics
- OPKO Health
- Miraca Holdings
- Myriad Genetics
- Sonic Healthcare
- Cardio Diagnostics Holdings, Inc
- Veravas
- BPGbio, Inc.
- Fulgent Genetics
- Alveo Technologies, Inc.
- S.R.L. Diagnostics
Sources
Primary Research Interviews from the following stakeholders:
Stakeholders:
- Interviews with laboratory directors, diagnostic technology developers, molecular diagnostic specialists, and healthcare providers specializing in esoteric and specialized testing.
- Specific stakeholders: Lab managers, molecular biology researchers, heads of pathology labs, senior executives at independent reference labs, clinical trial managers, and diagnostic assay developers.
Databases:
- PubMed
- National Center for Biotechnology Information (NCBI)
- American Clinical Laboratory Association (ACLA) Database
- Centers for Disease Control and Prevention (CDC) Health Databases
Magazines:
- Clinical Lab Products Magazine
- MedTech Dive
- Diagnostic World
- BioSpace News
Journals:
- Journal of Molecular Diagnostics
- Clinical Chemistry Journal
- Archives of Pathology & Laboratory Medicine
- Journal of Clinical Microbiology
Newspapers:
- The New York Times (Health Section)
- The Guardian (Health and Science Section)
- The Washington Post (Health)
- Financial Times (Healthcare Innovation)
Associations:
- American Association for Clinical Chemistry (AACC)
- College of American Pathologists (CAP)
- Association for Molecular Pathology (AMP)
- International Federation of Clinical Chemistry and Laboratory Medicine (IFCC)
Public Domain Sources:
- World Health Organization (WHO)
- U.S. Food and Drug Administration (FDA)
- National Institutes of Health (NIH)
- World Bank Healthcare Database
Proprietary Elements:
- CMI Data Analytics Tool and Proprietary CMI Repository of diagnostic, molecular, and esoteric testing information collected over the last 8 years.
Definition: Esoteric testing refers to specialized diagnostic tests that analyze biomarkers, DNA, RNA, proteins and other substances to provide information about rare conditions, personalized therapy selection, and disease monitoring. These tests require advanced instrumentation and are performed at dedicated reference labs by trained personnel. Esoteric tests guide treatment decisions for complex cases and conditions where conventional lab tests are inadequate. They include genomic testing, proteomic analysis, flow cytometry, and molecular diagnostic assays for cancers, neurological disorders, inherited diseases, and infectious diseases. Esoteric testing enables early diagnosis, targeted therapy selection and precise disease management
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients