Electronic Clinical Outcome Assessment (eCOA) Market Size and Trends
Global electronic clinical outcome assessment (eCOA) market is estimated to be valued at USD 2.00 Bn in 2025 and is expected to reach USD 5.36 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 15.1% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The electronic clinical outcome assessment (eCOA) market is expected to witness high growth over the forecast period due to the rising adoption of clinical trial digitalization solutions. eCOA solutions help in improving the quality of clinical trial data collection processes by reducing errors and streamlining workflows. They offer features such as centralized data management, remote data collection, and real-time data assessment. The COVID-19 pandemic has further accelerated the demand for digital clinical outcome assessment tools as they enable decentralized clinical trials and remote patient monitoring. The increasing R&D expenditure of pharma/biopharma companies and growing support from regulatory agencies are also contributing to the revenue opportunities in the electronic clinical outcome assessment (eCOA) market.
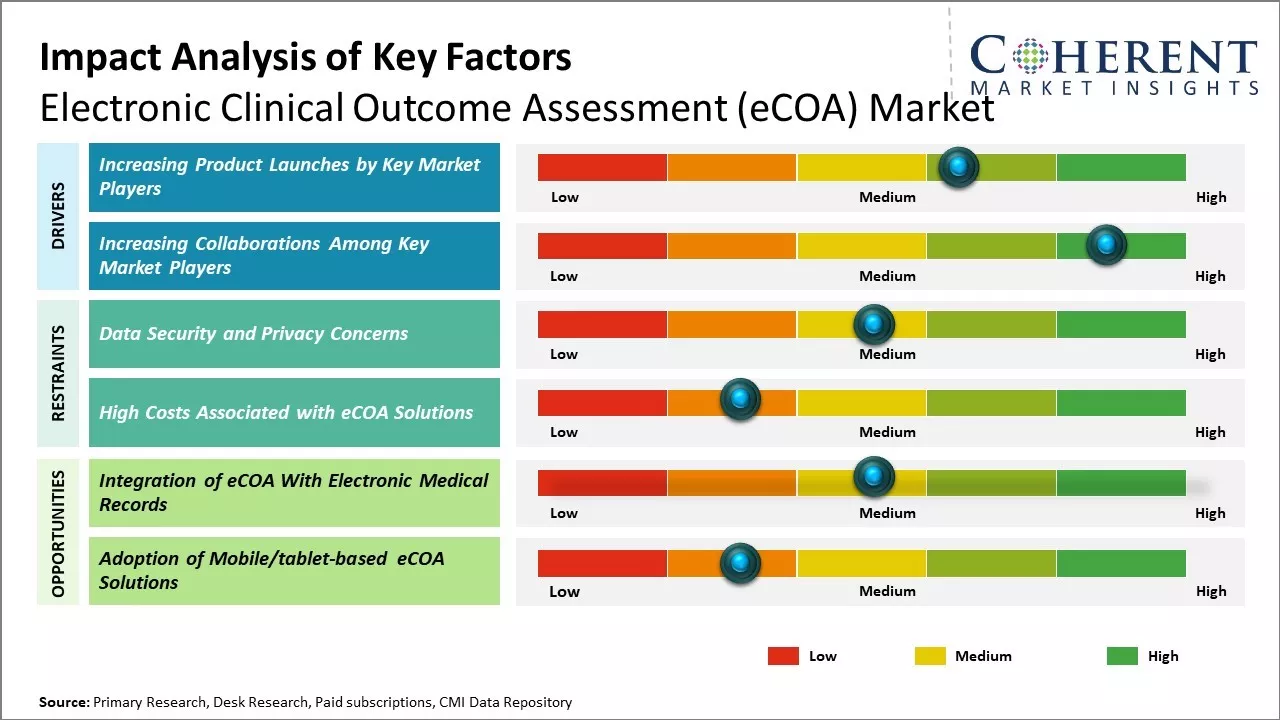
Market Driver – Increasing Product Launches by Key Market Players
Increasing adoption of organic growth strategies such as product launches by key market players is expected to drive the market growth over the forecast period. For instance, in December 2023, Obvio Health USA, Inc., a global digital clinical trials company, announced the launch of electronic clinical outcome assessment (eCOA) solution, seamlessly integrating advanced study design technology with scientific and clinical services to deliver more robust outcomes for trial sponsors.
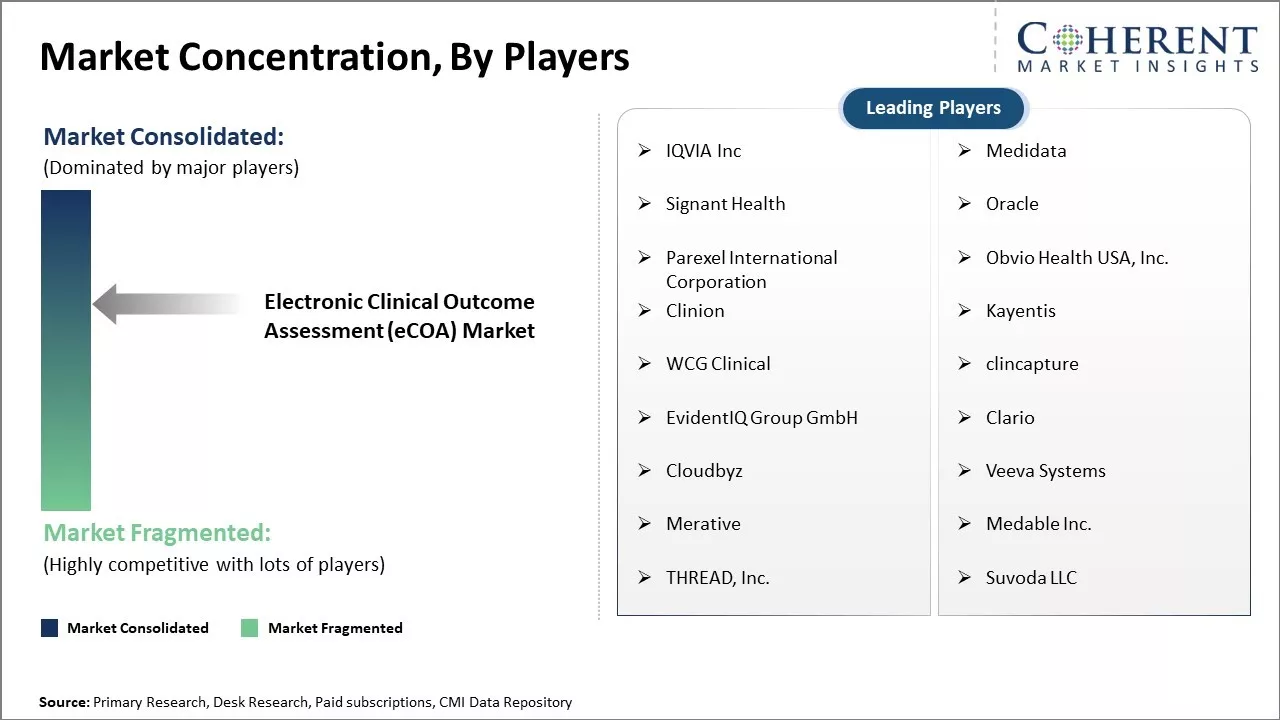
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Market Driver – Increasing Collaborations Among Key Market PlayersIncreasing collaborations among key market players is expected to drive the market growth over the forecast period. For instance, on February 6, 2024, Medidata, a technology company, announced a collaboration with Sanofi to harness Medidata eCOA to deploy in vaccine studies. The collaboration will use an eDiary function within eCOA to create an eDiary library specific to Sanofi’s vaccines. This library will accelerate future study set-up times, improve efficiency, and increase data quality, while ensuring patients have access to eDiaries that are easy to use.
Key Takeaways of Analyst:
The electronic clinical outcome assessment (eCOA) market is poised to grow significantly over the next few years. Major drivers of adoption will be the need for faster clinical trials and the push for more patient-centric approaches in clinical research. As new protocols emphasize capturing outcomes directly from patients, eCOA solutions provide a promising way to efficiently collect and analyze patient-reported outcome measures. The market has responded with many new software platforms and specialized devices for electronic data collection.
North America currently dominates the electronic clinical outcome assessment (eCOA) market but Europe and Asia Pacific are expected to witness faster growth. Stringent regulatory requirements in the U.S. and Canada have led to earlier adoption there. However, an increasing number of clinical trials are being conducted in emerging markets to take advantage of larger patient pools. This is expected to fuel the demand for eCOA in regions like India and China.
While upfront costs associated with implementing new technology solutions poses a challenge for smaller biopharma companies and clinical research organizations, the increased productivity, accuracy and transparency afforded by eCOA should help justify those investments over the long term. Data standardization across platforms remains an ongoing area of development as well.
Market Challenges: Data Security and Privacy Concerns
Data security and privacy concerns are a major restraint on the growth of the electronic clinical outcome assessment (eCOA) market. Patients and healthcare providers are hesitant to adopt eCOA solutions as they collect and store highly sensitive personal health information. Any breach of eCOA systems or unauthorized access to patient data can compromise privacy and have severe legal and financial repercussions. This act as a significant roadblock for many organizations who are wary of the risks. Compliance with stringent data privacy regulations also poses compliance challenges for eCOA providers. Regions and countries have different laws regarding collection, storage, and sharing of personal health information. International trials and multi-country studies involve navigating a complex web of global and local privacy norms. Ensuring compliance across diverse compliance landscapes increases costs and efforts for eCOA firms. Many lack adequate expertise to address the intricacies of privacy rules in different jurisdictions. The regulatory difficulties make it tough for companies to scale operations beyond domestic markets.
Market Opportunities: Integration of eCOA With Electronic Medical Records
The integration of electronic Clinical Outcome Assessments (eCOA) with Electronic Medical Records (EMRs) presents a major opportunity for the growth of the eCOA market going forward. eCOA solutions allow clinical trial data to be captured electronically by trial subjects themselves, replacing traditional paper-based methods. However, integrating this patient-centric data with Electronic Medical Record systems could help realize the full potential of eCOA. At present, eCOA data is often collected and stored separately from other clinical information. Integrating it with EMRs would provide clinicians a more comprehensive view of patients' health by combining clinical ratings, symptoms, and quality-of-life data with other medical records. This unified view would enhance clinical decision making and improve healthcare outcomes. It may also help speed clinical research by facilitating real-world data collection and enabling easier data extraction for trials directly from routine medical encounters documented in EMRs.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
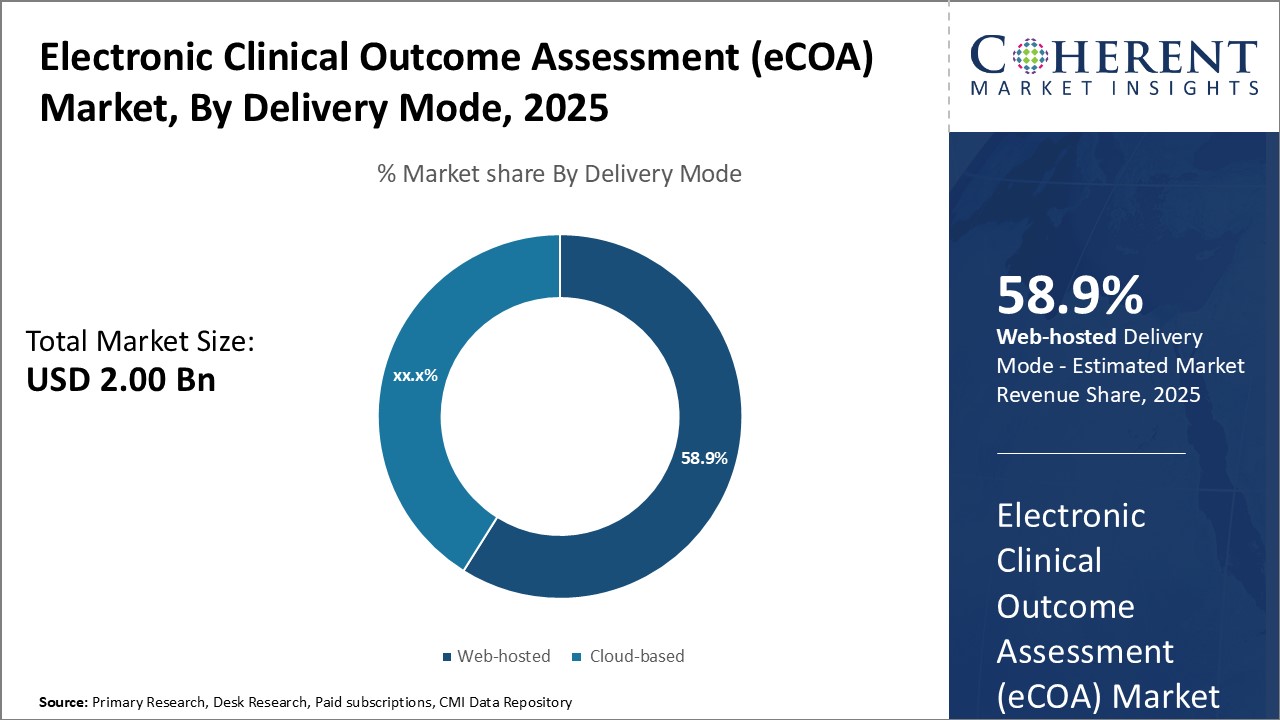
Insights, By Delivery Mode: Cost-effectiveness Fuels the Adoption of Web-hosted Delivery ModeDelivery mode segment is sub-segmented into web-hosted and cloud-based. The web-hosted sub-segment is estimated to hold 58.9% of the market share in 2025, due to its cost-effectiveness relative to cloud-based solutions. Web-hosted platforms involve lower upfront infrastructure investments for end users as they do not require purchasing and maintaining servers. This reduces capital expenditures for pharmaceutical companies, CROs, and healthcare providers. The subscription-based payment model of web-hosted eCOA also makes costs more predictable versus unpredictable expenses of cloud deployments that scale with usage and storage requirements. Ease of deployment and implementation is another factor supporting the growth of the web-hosted segment. Web applications are quickly installed on existing internet connections and devices without complex setup procedures. This enables faster clinical trial startups and data collection versus cloud-based systems requiring IT personnel for provisioning computing resources. The simplicity of web-hosted eCOA also means lower training needs for site staff and patients to use the systems. Regulatory concerns over data security and residency are less of an issue for web-hosted platforms versus cloud. Data hosted on provider servers within a country addresses domestic privacy laws, minimizing compliance risks. While cloud migration is increasing, regulatory clarity around international data transfer remains limited in some jurisdictions, supporting the preference for web-hosted approaches. Overall, the cost-effectiveness and implementation advantages of web-hosted eCOA relative to cloud-based solutions continue powering its leadership position within the delivery mode segment.
Insights, By Approach: Patient Centrality Drives PRO Approach Adoption
Approach segment is sub-segmented into patient-reported outcome (PRO), clinician-reported outcome (ClinRO), observer-reported outcome (ObsRO), and performance outcome (PerfO). The patient-reported outcome (PRO) sub-segment is estimated to hold 30.4% of the market share in 2025 due to the patient-centricity of such data. PRO tools empower patients to directly report on perceptions of symptoms, functional status, quality of life, satisfaction with care, and other experiences. This shifting of data collection away from clinicians aligns with growing patient autonomy in healthcare decision making. Pharmaceutical sponsors also value PRO measures as meaningful clinical trial endpoints reflecting a treatment's true impact from the patient perspective. The self-reported nature of PRO data makes it less susceptible to observer bias versus approaches involving clinicians or others. Patients are arguably the most qualified assessors of their own experiences and status. Self-reported tools also improve data quality by reducing missed interviews or assessments compared to options dependent on site staff resources. This supports more complete and consistent data capture central to clinical trials. Increased adoption of patient-centricity regulations worldwide also drives PRO leadership. Regulators now mandate incorporating patient inputs into drug evaluation and development processes. PRO-reported impacts directly fulfill such requirements in a way observer-based tools cannot. Overall, the patient-focused, unbiased, and compliant attributes of PRO approaches underpin its primacy within the eCOA assessment segment.
Insights, By End User: Access to Diverse Customers Fuels Pharma/Biotech End User Dominance
End user segment is sub-segmented into pharmaceutical & biotechnology, hospitals/healthcare providers, contract research organizations, and others. The pharmaceutical & biotechnology sub-segment is estimated to hold 38.7% of the market share in 2025, due to their diverse customer base of clinical trial sponsors. Pharma/biotech providers develop and validate eCOA delivery platforms targeting the specific needs of drug developers. These include configurable study designs, integrated randomization, and drug supply management capabilities, flexible data export formats, and statistical programming interfaces. Access to the entire spectrum of drug developers from small biotechs to large pharmaceutical firms allows eCOA providers to achieve considerable scale compared to other end users with narrower customer sets. This volume translates to greater resources for platform enhancements, expanded technical support staff, and larger sales/marketing teams. It also facilitates competitive pricing through economy of scale benefits passed on to customers. Strict regulatory requirements for clinical data quality and integrity in drug trials further drive pharmaceutical use of specialized eCOA platforms. Relative to other end users, pharma/biotech sponsors face the most risk from inspection findings related to inappropriate or invalidated efficacy/safety results. Qualified and validated eCOA offerings fundamentally designed for regulated environments give sponsors enhanced assurance versus less regulated end users. Overall, the higher demand and compliance needs of pharmaceutical/biotechnology customers underly their primacy within the end user segment of the electronic clinical outcome assessment market.
Regional Insights
Need a Different Region or Segment? Download Free Sample
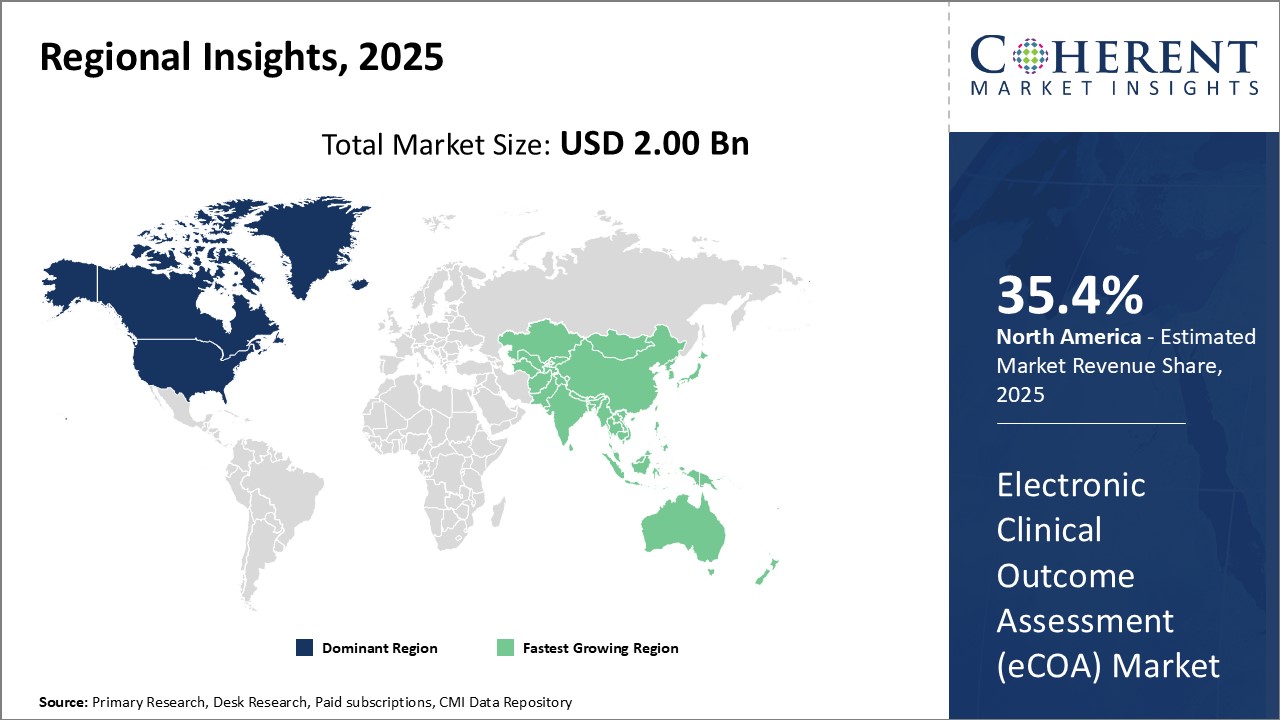
North America remains the dominant region in the global electronic clinical outcome assessment (eCOA) market and is estimated to hold 35.4% of the market share in 2025. The strong presence of major eCOA providers in the U.S. along with strategic collaborations between eCOA developers and CROs conducting global clinical trials has made North America the dominant region. The region also has a highly developed pharmaceutical industry that is quick to adopt and innovative technologies like eCOA. A majority of the larger global clinical trials are initiated in the U.S., which generates significant demand for eCOA solutions to digitally collect patient-reported outcomes.
Asia Pacific has emerged as the fastest growing regional market for eCOA in recent years. Countries like China, India, Japan, and South Korea are aggressively boosting their pharmaceutical manufacturing and clinical research capabilities. This has created substantial opportunities for eCOA providers looking to penetrate new markets. The availability of low-cost clinical trials, growing expertise of CROs, and quick regulatory approvals have led more global pharmaceutical sponsors to conduct some portions of international multi-center trials in Asia Pacific. This is driving the need for eCOA to collect and manage patient data electronically across sites. Governments in the region also provide funding and other incentives to develop domestic life sciences industries and tech infrastructure to compile medical data digitally. These factors have significantly raised demand and uptake of eCOA solutions in Asia Pacific.
Market Report Scope
Electronic Clinical Outcome Assessment (eCOA) Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 2.00 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 15.1% | 2032 Value Projection: | USD 5.36 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
IQVIA Inc, Medidata, Signant Health, Oracle, Parexel International Corporation, Obvio Health USA, Inc., Clinion, Kayentis, WCG Clinical, ÑlinÑapture, EvidentIQ Group GmbH, Clario, Cloudbyz, Veeva Systems, Merative, Medable Inc., THREAD, Inc., and Suvoda LLC |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Electronic Clinical Outcome Assessment (eCOA) Industry News
- On January 8, 2024, Medable Inc., a technology provider for clinical trials, introduced a new automation technology applied across its clinical trials platform that is designed to cut standard trial build timelines. By automating manual tasks such as testing, Medable Inc.'s new technology aims to save time and remove electronic clinical outcomes assessment (eCOA) from the critical path to trial go-live.
- In October 2023, Clario, a healthcare research technology company, announced a strategic partnership with Trial Data, a leading decentralized clinical trial (DCT) service provider, to bring innovative decentralized clinical trials solutions to the Chinese market and advance patient-centric clinical trials in China. This collaboration combines vast DCT experience and eCOA solutions, along with deep clinical trial operations experience in China, resulting in increased capabilities and flexibility in clinical trial strategies for sponsors supporting clinical trials in China.
- In September 2023, THREAD, Inc., a medical technology company, announced the launch of a suite of new complex electronic clinical outcome assessment (eCOA) features, including an expanded global library, to its decentralized research platform serving global researchers
- In December 2022, Suvoda LLC, an eCOA clinical trial technology company, announced that it had introduced the electronic clinical outcome assessments (eCOA) design toolkit, as the solution moves to the Early Adopter programme’s second phase. The toolkit is created to integrate smoothly with Suvoda IRT and eConsent, and also address the historical inadequacies that still impact eCOA.
*Definition: An electronic Clinical Outcome Assessment (eCOA) is a digital version of a Clinical Outcome Assessment (COA) that measures and records how a patient is feeling or functioning during a clinical trial. It is used to evaluate the efficacy of a health intervention by capturing patient-reported outcomes directly from patients, caregivers, and clinicians through various technologies like smartphones, tablets, computers, and interactive voice response systems. eCOA encompasses different types of assessments, including Patient Reported Outcomes (PRO), Clinical Reported Outcomes (ClinRO), Observer Reported Outcome (ObsRO), Performance Outcomes (PerfO), and Electronic Patient-Reported Outcome (ePRO). This technology offers benefits such as improved data quality, efficient data collection, real-time monitoring, and the ability to streamline processes in clinical trials.
Market Segmentation
- Delivery Mode Insights (Revenue, USD Bn, 2020 - 2032)
- Web-hosted
- Cloud-based
- Approach Insights (Revenue, USD Bn, 2020 - 2032)
- Patient-reported Outcome (PRO)
- Clinician-reported Outcome (ClinRO)
- Observer-reported Outcome (ObsRO)
- Performance Outcome (PerfO)
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Pharmaceutical & Biotechnology
- Hospitals/Healthcare Providers
- Contract Research Organizations
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- IQVIA Inc
- Medidata
- Signant Health
- Oracle
- Parexel International Corporation
- Obvio Health USA, Inc.
- Clinion
- Kayentis
- WCG Clinical
- сlinсapture
- EvidentIQ Group GmbH
- Clario
- Cloudbyz
- Veeva Systems
- Merative
- Medable Inc.
- THREAD, Inc.
- Suvoda LLC
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
