Downstream Processing Market Size and Forecast – 2025 to 2032
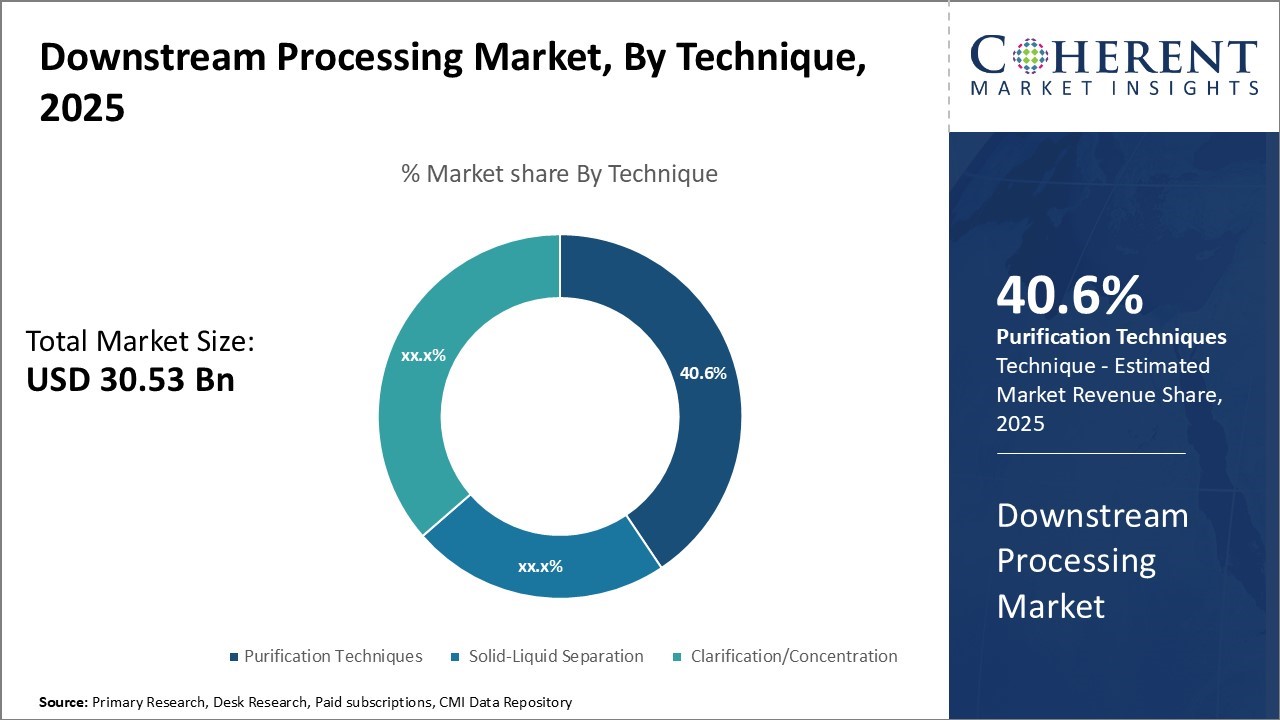
The Global Downstream Processing Market is estimated to be valued at USD 30.53 Bn in 2025 and is expected to reach USD 91.12 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 16.9% from 2025 to 2032.
Key Takeaways
- By Technique, Purification Technique hold the largest market share of 40.6% in 2025 owing to the rising demand for biopharmaceuticals.
- By Product, Chromatography Columns and Resins expected to hold largest market share of 40.71% in 2025 owing to the increasing demand for monoclonal antibodies and biosimilars.
- By Application, Antibodies Production acquired the prominent market share of 30.72% in 2025 owing to the rising global demand for monoclonal antibody (mab) therapies.
- By End User, Biopharmaceutical Companies hold the largest market share in 2025 owing to the strong growth in biologic drug pipelines.
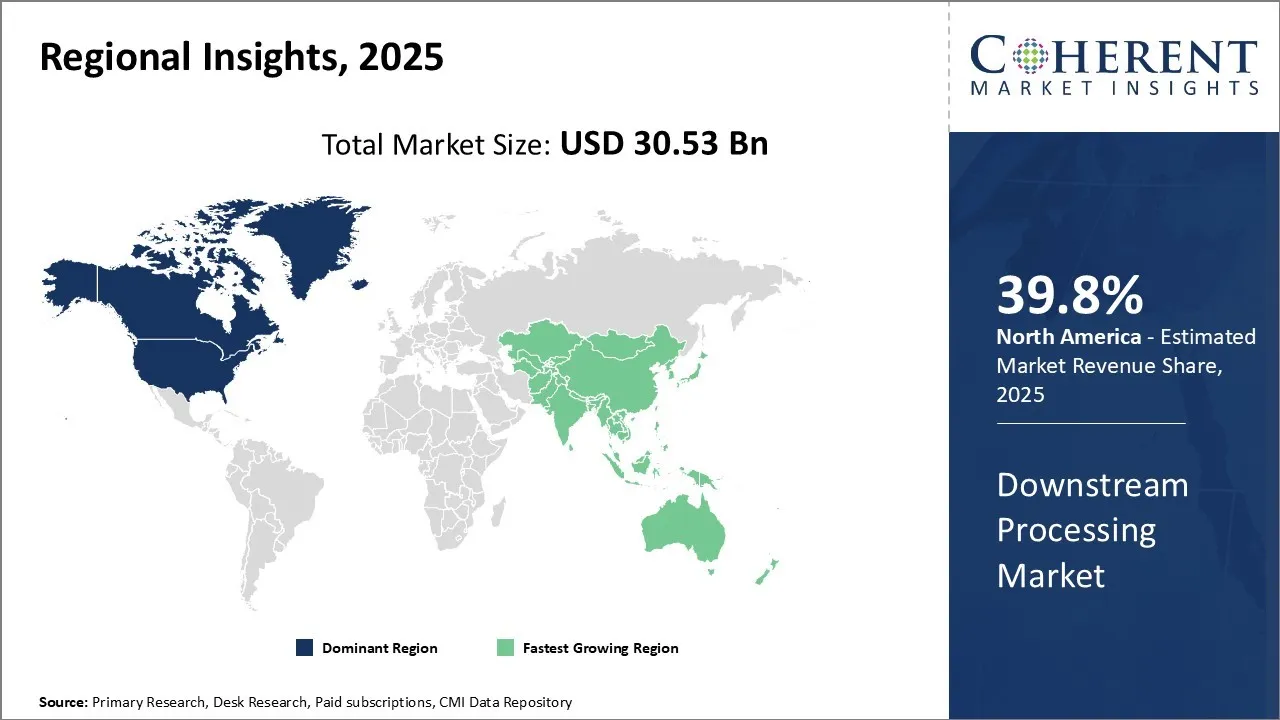
- By Region, North America dominates the overall market with an estimated share of 39.8% in 2025 owing to the robust R&D infrastructure & high investments.
Market Overview
The downstream processing market plays a vital role in biopharmaceutical manufacturing by enabling the purification, separation, and formulation of biologics such as monoclonal antibodies, vaccines, and recombinant proteins. Expanding biologics pipelines, rising demand for high-purity therapeutics, and stringent regulatory quality standards drive market growth. Ongoing advancements in chromatography, filtration, and single-use technologies, together with increased outsourcing to contract manufacturing organizations, further support the market’s growing global adoption.
Current Events and Its Impact on the Downstream Processing Market
|
Current Events |
Description and its impact |
|
Geopolitical and Regulatory Developments |
|
|
Technological Innovations and Industry Advances |
|
|
Environmental and Sustainability Trends |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Downstream Processing Market Insights, By Technique - Purification Technique contribute the highest share of the market owing to its increased R&D and bioprocessing investments
Purification Technique hold the largest market share of 40.6% in 2025. Purification techniques actively propel the downstream processing market by meeting the growing complexity of modern biologics and ensuring consistent product quality. Biopharmaceutical manufacturers use advanced purification methods to remove process-related impurities efficiently while maintaining product integrity. Increasing production of complex molecules, higher expectations for reliable processes, and the need to enhance yield and operational efficiency drive manufacturers to adopt innovative purification solutions, including highly selective, scalable, and automation-ready technologies in commercial manufacturing workflows.
For instance, in August 2025, Swedish cleantech startup Adsorbi launched its first pilot plant to manufacture bio-based adsorbents for the air purification industry, positioning the company for commercial scaling and strategic partnerships.
Downstream Processing Market Insights, By Product - Chromatography Columns and Resins contribute the highest share of the market owing to its adoption of pre-packed and automated systems
Chromatography Columns and Resins expected to hold largest market share of 40.71% in 2025. Chromatography columns and resins actively propel the downstream processing market by providing precise and efficient separation of high-value biologics during purification. As biopharmaceutical products grow more complex, manufacturers rely on advanced resin chemistries and durable column designs to achieve high selectivity and consistent results. The drive to enhance product recovery, reduce processing time, and maintain uniform batch quality motivates manufacturers to adopt high-capacity, scalable, and ready-to-use chromatography solutions throughout research, clinical, and commercial production stages.
For instance, in May 2025, BioChromatographix International (BCI), which recently launched in Singapore, is developing a new class of next-generation monolithic chromatography media to advance downstream processing for gene therapy, cell therapy, and advanced biologics manufacturing.
Downstream Processing Market Insights, By Application - Antibodies Production contribute the highest share of the market owing to its growth of next-generation antibody formats
Antibodies Production acquired the prominent market share of 30.72% in 2025. The production of antibodies actively drives the downstream processing market by creating strong demand for efficient purification and quality control methods. As therapeutic antibodies, including monoclonal and bispecific types, play a central role in treating cancer, autoimmune, and infectious diseases, manufacturers focus on processes that maintain purity, stability, and high yield. The development of complex antibody formats and strict regulatory requirements prompts manufacturers to implement advanced chromatography, filtration, and polishing techniques, fostering innovation and widespread adoption of specialized downstream processing solutions in both clinical and commercial manufacturing.
In September 2024, MilliporeSigma, the life sciences division of Merck, introduced a single-use reactor for manufacturing antibody drug conjugates (ADCs). The system employs disposable, sterile, and pre-validated components, which minimize the risk of cross-contamination between batches.
Downstream Processing Market Insights, By End User - Biopharmaceutical Companies contribute the highest share of the market owing to its adoption of advanced and single-use technologies
Biopharmaceutical companies actively drive the downstream processing market by creating strong demand for efficient, high-quality purification solutions. As they expand pipelines of monoclonal antibodies, vaccines, and recombinant proteins, they focus on implementing robust downstream processes to maintain product safety, consistency, and yield. Their investments in advanced technologies, automation, and scalable systems, combined with increasing outsourcing to contract manufacturing organizations, accelerate the adoption of innovative DSP solutions. This proactive approach to process optimization enhances the market’s overall growth and sophistication.
In April 2025, AustinPx, a CDMO specializing in bioavailability enhancement for oral small molecule drugs, has launched the KinetiLease™ program, enabling pharmaceutical companies to lease its KinetiSol™ research-scale equipment.
Regional Insights
To learn more about this report, Download Free Sample
North America Downstream Processing Market Trends
North America dominates the overall market with an estimated share of 39.8% in 2025. In North America, strong biopharmaceutical activity and continuous technological innovation actively shape the downstream processing market. Companies in the region adopt single-use systems, continuous processing, and automated purification solutions to boost efficiency and minimize contamination risks. Advanced manufacturing infrastructure, significant R&D investment, and supportive regulatory frameworks enable rapid implementation of cutting-edge downstream technologies. Additionally, rising production of biologics, vaccines, and biosimilars, coupled with increased outsourcing to contract manufacturing organizations, actively drives market growth and enhances operational sophistication.
For instance, in November 2025, Global Battery Materials Corp. launched as a vertically integrated critical minerals and technology company. GB Materials operates a pilot plant and research center in Siheung, supporting its downstream operations with advanced technological capabilities.
Europe Downstream Processing Market Trends
the downstream processing market grows steadily due to substantial investment in biopharmaceutical research and the adoption of advanced purification technologies in Europe. Companies increasingly utilize single-use systems, high-capacity chromatography, and automated workflows to improve efficiency and maintain high product quality. Supportive regulatory frameworks, combined with a focus on biosimilars and innovative biologics, encourage the implementation of scalable and adaptable downstream solutions. In addition, partnerships with contract manufacturing organizations and ongoing process optimization continue to expand and modernize the region’s downstream processing sector.
United States Downstream Processing Market Trends
In the United States, the strong biopharmaceutical industry and ongoing technological advancements actively drive the downstream processing market. Companies implement single-use systems, continuous processing, and automated purification technologies to increase efficiency, reduce contamination, and maintain high product quality. Extensive R&D investment, advanced manufacturing infrastructure, and supportive regulatory frameworks allow manufacturers to adopt advanced downstream solutions rapidly. Moreover, companies expand biologics, vaccine, and biosimilar production and partner with contract manufacturing organizations, actively growing the market and strengthening the U.S.’s leadership in global downstream processing.
United Kingdom Downstream Processing Market Trends
In the United Kingdom, a strong focus on biopharmaceutical research and innovation actively drives the downstream processing market. Companies adopt advanced purification technologies, such as single-use systems, high-capacity chromatography, and automated workflows, to improve process efficiency and ensure product quality. Supportive regulatory frameworks and increasing emphasis on biologics, vaccines, and biosimilars encourage manufacturers to implement scalable and flexible downstream solutions. Furthermore, partnerships with contract manufacturing organizations and ongoing process optimization actively expand and modernize the UK’s downstream processing industry.
End-user Feedback and Unmet Needs in the Downstream Processing Market
- Demand for Higher Process Efficiency: End users report a need for faster and more efficient downstream processes to reduce production time and costs. Manufacturers seek technologies that streamline purification, minimize bottlenecks, and optimize yield, enabling quicker scale-up from R&D to commercial production while maintaining product quality and consistency across batches.
- Need for Cost-Effective Solutions: Many end users highlight the high cost of downstream processing consumables, equipment, and labor. Companies require solutions that reduce operational expenses without compromising purity, recovery, or compliance. Affordable, scalable systems and reusable or high-capacity consumables remain unmet needs to improve cost-efficiency in biologics manufacturing.
- Requirement for Scalability and Flexibility: End users increasingly demand downstream processes that can adapt to varying production scales, from clinical batches to commercial volumes. Flexible, modular, and easily scalable systems are required to support diverse biologics portfolios and personalized therapies, ensuring consistent quality while accommodating production growth and changing market needs.
Downstream Processing Market Trend
Adoption of Single-Use Technologies
The market is increasingly shifting toward single-use systems, including disposable bioreactors, filtration units, and chromatography columns. These technologies reduce cross-contamination risks, lower cleaning and validation requirements, and provide flexibility for small- to large-scale manufacturing. End users prefer single-use solutions for their efficiency, cost-effectiveness, and ability to accelerate process timelines, particularly in multi-product facilities and contract manufacturing organizations focusing on biologics and personalized therapies.
Continuous Processing Implementation
Continuous downstream processing is gaining momentum as companies seek to improve productivity and reduce footprint. Integrating continuous chromatography, filtration, and buffer management allows manufacturers to enhance yield, reduce operational costs, and achieve consistent product quality. This trend supports faster scale-up, minimizes downtime, and aligns with process intensification strategies in both clinical and commercial manufacturing of complex biologics.
Downstream Processing Market Opportunity
Growth in Biosimilars and Generic Biologics
The global push for biosimilars and generic biologics creates opportunities for downstream processing providers. These products require efficient, reproducible, and cost-effective purification processes to achieve regulatory comparability with reference drugs. Companies offering flexible DSP platforms, pre-packed columns, and high-throughput purification solutions can help biopharmaceutical manufacturers reduce production costs, improve yield, and accelerate time-to-market for biosimilars and emerging biologics.
Market Report Scope
Downstream Processing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 30.53 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.9% | 2032 Value Projection: | USD 91.12 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Merck KGaA, Thermo Fisher Scientific Inc., GE Healthcare, Sartorius Stedim Biotech S.A., Repligen Corporation, Pall Corporation, M Company, Eppendorf AG, Agilent Technologies, Inc., Waters Corporation, Shimadzu Corporation, Bio-Rad Laboratories, Inc., Avantor Performance Materials, LLC, Tosoh Corporation, Column Technology Inc., Purolite, W.R. Grace & Co., Asahi Kasei Corporation, Novasep Holding S.A.S, and Boehringer Ingelheim |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Analyst Opinion (Expert Opinion)
- The downstream processing market is no longer a secondary operational concern but has become the strategic fulcrum of biopharmaceutical manufacturing excellence. Purification by chromatography alone commanded nearly USD 17.5 Bn in revenue in 2024, underscoring its indispensability in capturing high‑value biologics with requisite purity standards. Notably, North America retained dominance with over 40% market share, reflecting its advanced infrastructure and R&D intensity that forces innovation in purification, filtration, and single‑use integration.
- Critically, the pace of innovation is not incremental but transformative. The emergence of high‑throughput systems, such as Cytiva’s HiScreen Fibro PrismA offering a reported 20‑fold productivity increase in mAb purification, illustrates that vendors who innovate along efficiency vectors will reshape operational economics and facility footprints.
- Additionally, industry patterns reveal that downstream processing decisions now directly correlate with time‑to‑clinic and commercial viability. Contract manufacturers and biopharmaceutical firms are integrating real‑time analytics and continuous polishing steps to reduce variability and enhance batch consistency, a shift evident in the increasing uptake of hybrid systems that balance stainless‑steel robustness with single‑use flexibility.
Market Segmentation
- Technique Insights (Revenue, USD Bn, 2020 - 2032)
- Purification Techniques
- Solid-Liquid Separation
- Clarification/Concentration
- Product Insights (Revenue, USD Bn, 2020 - 2032)
- Chromatography Columns and Resins
- Filters
- Evaporators
- Centrifuges
- Other Products
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Antibodies Production
- Vaccines Production
- Insulin Production
- Other Applications
- End User Insights (Revenue, USD Bn, 2020 - 2032)
- Biopharmaceutical Companies
- CMOs/CDMOs
- Research Institutes
- CROs
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Merck KGaA
- Thermo Fisher Scientific Inc.
- GE Healthcare
- Sartorius Stedim Biotech S.A.
- Repligen Corporation
- Pall Corporation
- M Company
- Eppendorf AG
- Agilent Technologies, Inc.
- Waters Corporation
- Shimadzu Corporation
- Bio-Rad Laboratories, Inc.
- Avantor Performance Materials, LLC
- Tosoh Corporation
- Column Technology Inc.
- Purolite
- W.R. Grace & Co.
- Asahi Kasei Corporation
- Novasep Holding S.A.S
- Boehringer Ingelheim
Sources
Primary Research interviews
- Interviews with biopharmaceutical manufacturing experts
- Discussions with process engineers and downstream processing specialists
- Feedback from quality control and regulatory professionals
Databases
- PubChem
- Protein Data Bank (PDB)
- ClinicalTrials.gov
- European Bioinformatics Institute (EBI)
- FDA Drug Approval Database
Magazines
- Genetic Engineering & Biotechnology News (GEN)
- BioProcess International
- Pharmaceutical Technology
Journals
- Journal of Chromatography A & B
- Biotechnology and Bioengineering
- Journal of Pharmaceutical Sciences
- Current Opinion in Biotechnology
- Trends in Biotechnology
Newspapers
- The New York Times (Science Section)
- The Guardian (Science Section)
- Nature News
Associations
- International Society for Pharmaceutical Engineering (ISPE)
- American Chemical Society (ACS)
- Biopharmaceutical Manufacturing Association (BPA)
- European Federation of Biotechnology (EFB)
Public Domain sources
- World Health Organization (WHO) publications
- U.S. Food and Drug Administration (FDA) guidelines
- European Medicines Agency (EMA) reports
- United Nations Industrial Development Organization (UNIDO) reports
Proprietary Elements
- CMI Data Analytics Tool
- Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients