Controlled Release Drug Delivery Market Size and Forecast – 2025 to 2032
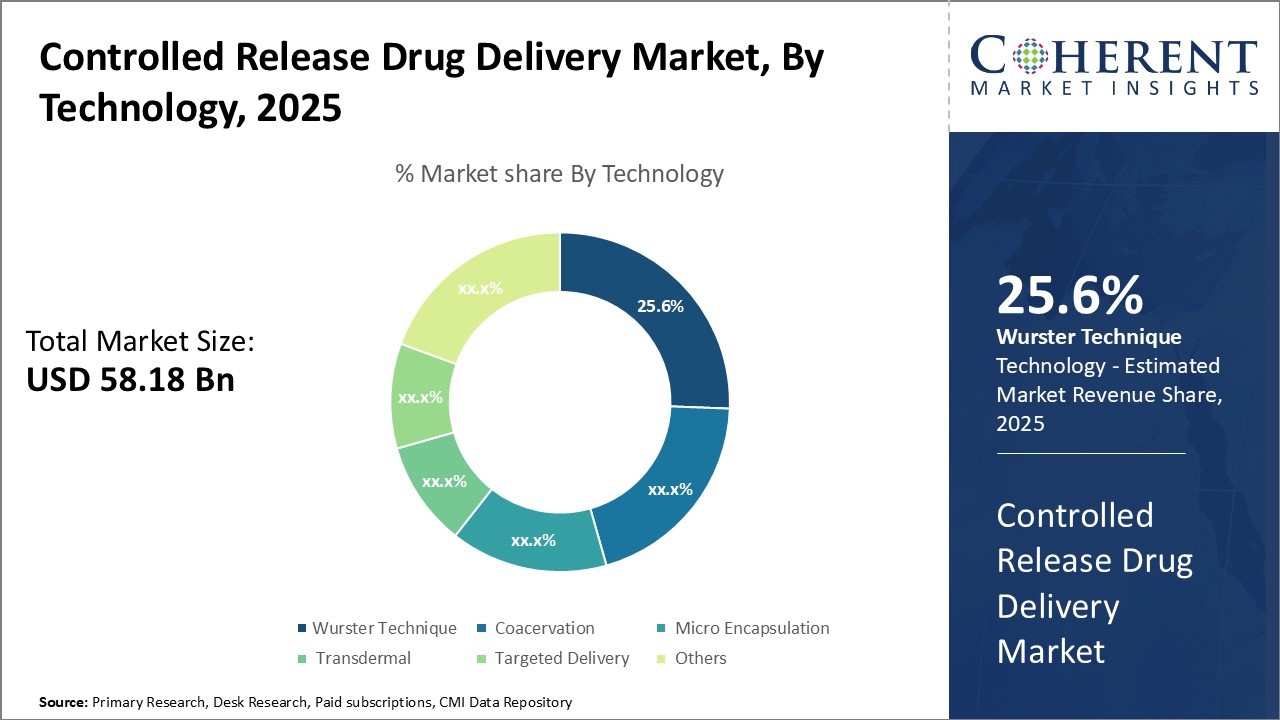
Global controlled release drug delivery market is estimated to be valued at USD 58.18 Bn in 2025 and is expected to reach USD 119.34 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.8% from 2025 to 2032.
Key Takeaways
- By Technology, Wurster Technique hold the largest market share of 25.6% in 2025 owing to the demand for improved patient compliance.
- By Therapeutic Area, Oncology acquired the prominent market share of 24.7% in 2025 owing to its need to reduce systemic toxicity of chemotherapy.
- By Application, Injectables expected to hold largest market share of 30.62% in 2025 owing to the demand for long‑acting formulations.
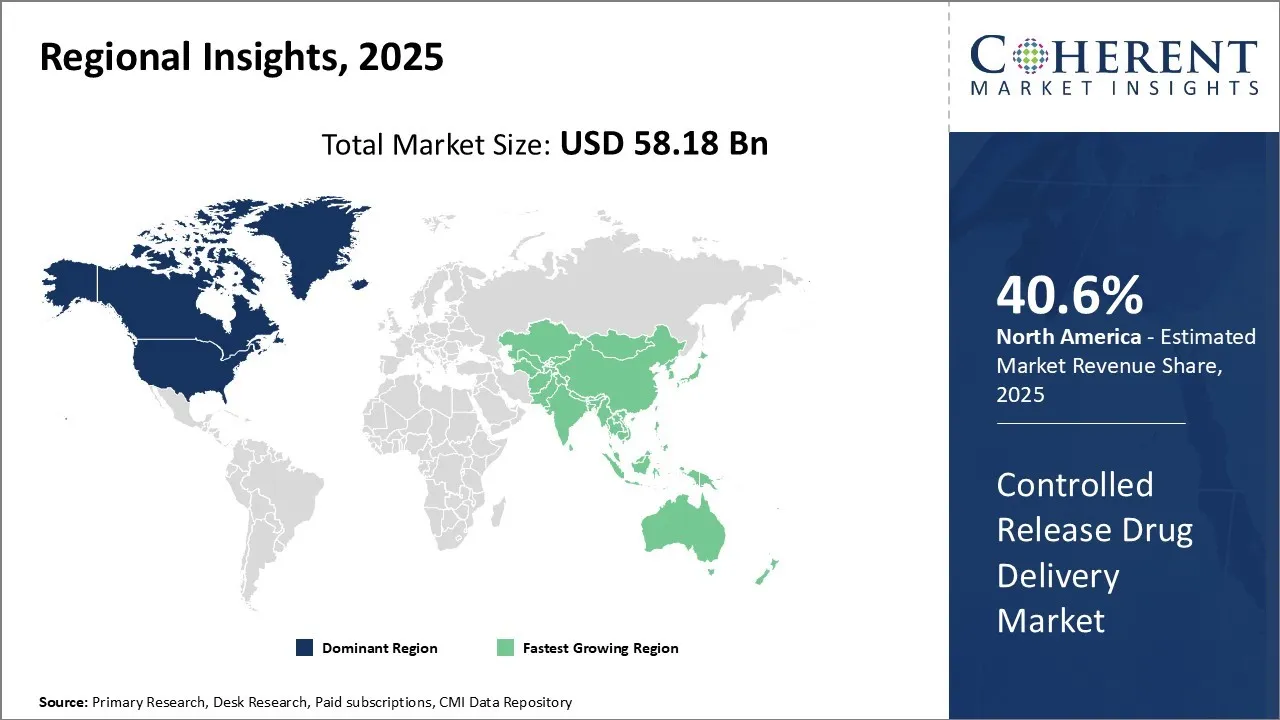
- By Region, North America dominates the overall market with an estimated share of 40.6% in 2025 owing to the high prevalence of chronic diseases.
Market Overview
Pharmaceutical companies are driving the controlled release drug delivery market demand by developing advanced therapies that enhance patient compliance, lower dosing frequency, and ensure consistent drug levels. The rising burden of chronic diseases, along with innovations in polymers and drug delivery systems, is fueling market expansion. Healthcare providers increasingly adopt controlled release technologies for treating oncology, diabetes, and central nervous system disorders. Supportive regulatory policies and growing pharmaceutical R&D efforts are also accelerating the market's growth across both developed and emerging regions.
Current Events and Its Impact
|
Current Events |
Description and its impact |
|
Technological Advancements in Nanotechnology |
|
|
Geopolitical and Trade Dynamics |
|
|
Pharmaceutical Industry Trends and Collaborations |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
End-user Feedback and Unmet Needs in the Controlled Release Drug Delivery Market
- Patient Convenience and Compliance: Users emphasize the need for formulations that reduce dosing frequency, enhancing convenience and adherence. Many seek easy-to-administer devices that integrate seamlessly into daily routines, minimizing discomfort and complexity. Improved patient-centric designs that address mobility or cognitive challenges remain an unmet need.
- Personalized Therapy and Dose Control: End-users demand customizable release profiles tailored to individual metabolic rates and conditions. Current one-size-fits-all approaches limit efficacy and safety. Adaptive delivery systems that can modulate drug release in real-time based on patient feedback are a critical unmet requirement.
- Stability and Shelf-Life Improvements: Patients and healthcare providers highlight concerns over drug stability during extended release. Unpredictable degradation affects therapeutic outcomes and safety. Innovations ensuring consistent release without compromising shelf life or storage conditions remain essential for market advancement.
Controlled Release Drug Delivery Market Insights, by Technology - Wurster Technique contribute the highest share of the market owing to its advances in polymer & coating materials
Wurster Technique hold the largest market share of 25.6% in 2025. This air suspension coating process can be applied to a wide range of pharmaceutical formulations, including tablets, pellets, granules, and powders, making it a highly versatile method for achieving controlled drug release. The specialized coating chamber of the Wurster Technique allows for efficient and uniform coating of the drug particles or dosage forms, resulting in better control over the drug release kinetics and the ability to engineer desired release profiles. Additionally, the Wurster Technique is easily scalable, making it suitable for both small-scale and large-scale pharmaceutical manufacturing operations. This scalability ensures consistent and reproducible production of controlled release drug products.
Controlled Release Drug Delivery Market Insights, by Therapeutic Area - Oncology contributes the highest share of the market owing to its targeted drug delivery
Oncology acquired the prominent market share of 24.7% in 2025. Conventional chemotherapy relies on high drug doses to target malignant tumor cells while also damaging healthy tissues. This lack of selectivity causes severe side effects that reduce patient quality of life and limit treatment effectiveness. Controlled release drug delivery helps optimize chemotherapy by maintaining therapeutic drug concentrations at tumor sites for extended periods. This prolonged exposure allows lower average doses to be administered while achieving the same anti-tumor effect. Sustained release at the target prevents drug concentrations from fluctuating above and below the effective threshold. Less systemic exposure means fewer side effects, enabling patients to better tolerate intensive, long-term therapies. Emerging targeted delivery technologies improves outcomes. Nanocarriers amenable to active tumor targeting help maximize intracellular drug accumulation in cancer cells.
In August 2025, N-Power Medicine launched the industry’s first Prospective External Control Arm (ProECA) platform to support pharmaceutical and biotechnology partners in oncology drug development.
Controlled Release Drug Delivery Market Insights, by Application - Injectable contribute the highest share of the market owing to its rising prevalence of chronic & infectious diseases
Injectables hold the largest controlled release drug delivery market share of 30.62% in 2025. As lifestyles become increasingly busy and medical needs more complex, consistent treatment adherence is challenging, thus, risking health complications. Controlled release injectable drug delivery empowers patients to maintain wellness independently. Long-acting intramuscular or subcutaneous depot formulations administer therapeutics gradually over weeks or months with a single administration. This eliminates the need for daily oral medication reminders and interrupts to routines. The convenience of infrequent dosing boosts compliance, a key factor determining treatment success rates. Controlled release injectables are also transforming care for conditions difficult to manage or sensitive to fluctuations in drug levels.
For instance, in April 2024, Eisai Co., Ltd. introduced the intravenous (IV) injection formulation of its antiepileptic drug Fycompa® (perampanel hydrate) in Japan.
Regional Insights
To learn more about this report, Download Free Sample
North America Controlled Release Drug Delivery Market Trends
North America dominates the overall market with an estimated share of 40.6% in 2025. Pharmaceutical and biotech companies in North America are transforming the controlled release drug delivery market by focusing on personalized medicine and patient-centered therapies. They are actively developing innovative platforms such as injectable depots, implantable, and smart drug systems. The region's advanced healthcare infrastructure, supportive regulatory environment, and high chronic disease burden drive this progress. Collaborations between research institutions and industry players continue to shape new solutions, while the rising preference for home-based care further accelerates the adoption of controlled release technologies.
For instance, in September 2024, PCI Pharma Services invested over USD 365 million in infrastructure to support clinical and commercial-scale final assembly and packaging of drug-device combination products, focusing on advanced injectable drug delivery systems.
Asia Pacific Controlled Release Drug Delivery Market Trends
Healthcare providers and pharmaceutical companies in Asia Pacific are accelerating the controlled release drug delivery market by modernizing systems and addressing the growing need for better treatment options. Governments and private investors are actively funding pharmaceutical innovation, promoting the development of advanced delivery technologies. Increased awareness of chronic disease care and broader access to healthcare services are boosting adoption. Local manufacturers are collaborating with global firms to introduce new therapies, while urban populations increasingly prefer convenient, long-acting treatment solutions throughout the region. For instance, Nelipak® Corporation, a leading global manufacturer of packaging solutions for medical devices, diagnostics, pharmaceutical drug delivery, and other demanding applications, is strengthening its commitment to directly serve customers in the Asia-Pacific region, as well as through its preferred partners.
United States Controlled Release Drug Delivery Market Trends
Pharmaceutical companies in the United States are driving the controlled release drug delivery market forward by developing cutting-edge technologies such as biodegradable implants, microspheres, and nanoparticle-based systems. Healthcare providers focus on improving patient adherence and minimizing side effects by providing more convenient dosing options. Government agencies actively support innovation and expedite product approvals through funding and regulatory initiatives. Collaborations between research institutions and industry players accelerate the creation of personalized therapies. Meanwhile, the rising incidence of chronic diseases fuels demand for sustained-release solutions that improve treatment outcomes and patient quality of life.
India Controlled Release Drug Delivery Market Trends
Pharmaceutical companies in India are rapidly expanding the controlled release drug delivery market by developing affordable and accessible formulations to address the growing demand for effective chronic disease management. The government actively promotes innovation through supportive policies and funding programs. Domestic manufacturers are collaborating with international firms to accelerate technology transfer and product development. Additionally, increasing healthcare awareness, urbanization, and improvements in healthcare infrastructure are driving wider adoption of advanced drug delivery systems throughout the country. For instance, in May 2025, Emcutix Biopharmaceuticals Ltd., together with Canada-based Mantra Pharma Inc.—both subsidiaries of Emcure Pharmaceuticals Ltd.— launched Ureaderm 10% & 20%, a urea-based moisturizing cream, across India. Backed by Emcure Pharmaceuticals, Emcutix is a dermatology company focused on delivering innovative skincare and aesthetic solutions in India.
Market Dynamics
Controlled Release Drug Delivery Trend
Precision Medicine Through Personalized Release Profiles
Controlled release systems are evolving toward highly personalized therapies that tailor drug release to individual patient needs. Advances in pharmacogenomics and biomarker monitoring enable customization of release kinetics, allowing drugs to be delivered at optimal times and doses for maximum efficacy. This trend supports better management of chronic diseases by considering patient-specific metabolism, lifestyle, and disease progression, ultimately improving treatment outcomes and reducing adverse effects through individualized, controlled delivery strategies.
Controlled Release Drug Delivery Opportunity
Expanding Chronic Disease Management
Controlled release drug delivery presents significant opportunities to enhance management of chronic illnesses like diabetes, cardiovascular diseases, and neurodegenerative disorders. By enabling steady, prolonged drug release, these systems improve symptom control and reduce the burden of frequent dosing. Innovations targeting sustained delivery can enhance patient quality of life and adherence, addressing the unmet needs of complex, lifelong therapies where maintaining consistent therapeutic levels is critical for long-term disease management.
Market Report Scope
Controlled Release Drug Delivery Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 58.18 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.8% | 2032 Value Projection: | USD 119.34 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Orbis Biosciences, Inc., Merck and Co., Inc., Alkermes plc, Johnson and Johnson, Coating Place, Inc., Corium International, Inc., Depomed, Inc., Pfizer, Inc, Aradigm Corporation, Capsugel., Abbott Laboratories, Roche Holdings AG, AstraZeneca, Baxter International Inc, Teva Pharmaceutical Industries Ltd., Bristol-Myers Squibb Company |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Controlled Release Drug Delivery Market News
- In January 2025, A pair of Husker researchers launched a startup to bring to market an innovative method for delivering therapeutics, gene-editing tools, plasmids, and more to targeted areas in the human body.
Analyst Opinion (Expert Opinion)
- The Controlled Release Drug Delivery market is at a pivotal juncture, driven less by incremental innovation and more by disruptive technological convergence. The integration of nanotechnology with biomaterials, exemplified by FDA-approved liposomal formulations like Doxil, clearly demonstrates the power of targeted, sustained release in minimizing systemic toxicity—a challenge long plaguing oncology treatments. Yet, despite these advances, the sector still underutilizes precision medicine tools. Current controlled release systems predominantly follow static release kinetics, which inadequately address the dynamic physiological variability seen in patients.
- The market urgently needs shift towards responsive, adaptive delivery platforms that leverage real-time biomarker data—this is where the true clinical value lies. For instance, glucose-responsive insulin delivery systems, still largely experimental, embody this future direction, promising to revolutionize diabetes management by autonomously modulating release in response to fluctuating blood sugar levels. The reluctance of pharmaceutical companies to aggressively pursue these complex, costly-to-develop systems stems partly from regulatory uncertainties and manufacturing challenges, but ignoring this potential risks obsolescence in an era rapidly advancing toward personalized therapeutics.
- Furthermore, the environmental footprint of drug delivery materials is gaining scrutiny. The widespread use of non-biodegradable polymers in implants and microspheres is unsustainable long-term. Emerging biodegradable alternatives like polylactic-co-glycolic acid (PLGA) have shown promise but require further refinement to balance release profiles with degradation kinetics. Companies investing in greener, biocompatible materials will not only meet regulatory and societal pressures but also capture an increasingly eco-conscious patient base.
Market Segmentation
- Technology Insights (Revenue, USD Bn, 2020 - 2032)
- Wurster Technique
- Coacervation
- Micro Encapsulation
- Transdermal
- Targeted Delivery
- Others
- Therapeutic Area Insights (Revenue, USD Bn, 2020 - 2032)
- Infectious Diseases
- Oncology
- Cardiology
- Neurology
- Autoimmune Diseases
- Others
- Application Insights (Revenue, USD Bn, 2020 - 2032)
- Metered Dose Inhalers
- Injectables
- Infusion Pumps
- Drug Eluting Stents
- Others
- Distribution Channel Insights (Revenue, USD Bn, 2020 - 2032)
- Hospital Pharmacy
- Retail Pharmacy
- Online Pharmacy
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players Insights
- Orbis Biosciences, Inc.
- Merck and Co., Inc.
- Alkermes plc
- Johnson and Johnson
- Coating Place, Inc.
- Corium International, Inc.
- Depomed, Inc.
- Pfizer, Inc
- Aradigm Corporation
- Capsugel
- Abbott Laboratories
- Roche Holdings AG
- AstraZeneca
- Baxter International Inc
- Teva Pharmaceutical Industries Ltd.
- Bristol-Myers Squibb Company
Sources
Primary Research interviews
- Industry experts from pharmaceutical companies
- R&D scientists specializing in drug delivery systems
- Regulatory affairs professionals
- Healthcare practitioners using controlled release therapies
- Academic researchers in pharmaceutical sciences
Databases
- PubMed
- ClinicalTrials.gov
- FDA Drug Approval Database
- World Health Organization (WHO) Global Health Observatory
- Scopus
Magazines
- Pharmaceutical Technology
- Drug Delivery and Development
- Pharma Manufacturing
- Pharmaceutical Executive
Journals
- Journal of Controlled Release
- International Journal of Pharmaceutics
- European Journal of Pharmaceutical Sciences
- Advanced Drug Delivery Reviews
- Journal of Pharmaceutical Sciences
Newspapers
- The New York Times (Health Section)
- The Guardian (Health & Science)
- Financial Times (Pharmaceutical News)
- The Wall Street Journal (Healthcare Section)
Associations
- Controlled Release Society (CRS)
- International Pharmaceutical Federation (FIP)
- American Association of Pharmaceutical Scientists (AAPS)
- European Federation for Pharmaceutical Sciences (EUFEPS)
Public Domain sources
- U.S. National Institutes of Health (NIH)
- Centers for Disease Control and Prevention (CDC)
- World Health Organization (WHO) reports
- Patent databases (USPTO, EPO)
Proprietary Elements
- CMI Data Analytics Tool, and Proprietary CMI Existing Repository of information for last 8 years
Share
Share
About Author
Vipul Patil is a dynamic management consultant with 6 years of dedicated experience in the pharmaceutical industry. Known for his analytical acumen and strategic insight, Vipul has successfully partnered with pharmaceutical companies to enhance operational efficiency, cross broader expansion, and navigate the complexities of distribution in markets with high revenue potential.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients