Closed System Transfer Device Market Size and Trends: 2025 to 2032
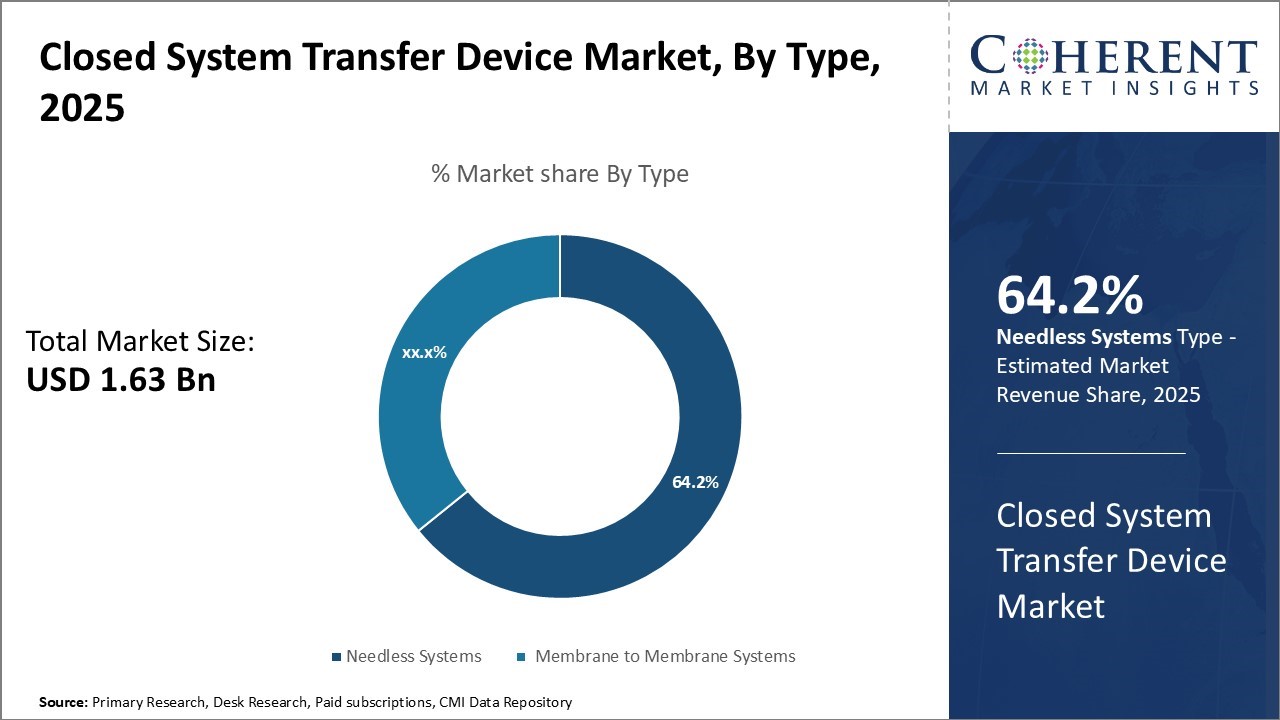
The closed system transfer device market is estimated to be valued at USD 1.63 Bn in 2025 and is expected to reach USD 4.82 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 16.7% from 2025 to 2032.
To learn more about this report, Download Free Sample
Key Takeaways
- Based on Type, the Membrane-to-Membrane segment is expected to lead the market with 64.2% share of the market in 2025, due to safe, aseptic drug transfer.
- Based on Closing Mechanism, the Luer Lock Systems segment is expected to hold the highest share of the market in 2025, owing to secure, universal syringe and IV compatibility.
- Based on Component, the Vial Access Devices segment is projected to capture the maximum share of the market in 2025, fueled by safe hazardous drug transfer.
- Based on Technology, the Diaphragm-Based Devices segment is projected to account for the greatest share of the market in 2025, driven by reliable, leak-free sealing.
- Based on End Use, the Hospitals & Clinics segment is expected to lead the market with largest share in 2025, due to high chemotherapy and CSTD use.
Market overview
The market is driven by the need to curb needlestick injuries and exposure to hazardous drugs. Closed system transfer devices provide an extra layer of safety compared to traditional methods during drug preparation and administration.
The closed system transfer device market is expected to witness significant growth over the forecast period. With increasing awareness about potential dangers of cytotoxic drug exposure and regulatory mandates to curb occupational hazards, healthcare providers are widely adopting closed drug transfer systems. Also, growing chemotherapy patient population and cancer cases worldwide is further expected to drive the demand for safer handling of hazardous drugs. Market players are also launching innovative product variants to better address needs across various therapeutic areas.
Current Events and Its Impact
|
Current Event |
Description and its Impact |
|
Regulatory Landscape Evolution |
|
|
Oncology and Pharmaceutical Industry Growth |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Closed System Transfer Device Market Insights, By Type - Lead for Safe, Aseptic Drug Transfer
In terms of type, membrane to membrane systems is expected contribute the highest share of the market owing 64.2% in 2025 to their superior safety features that reduce risk of exposure during drug preparation and administration. Membrane to membrane systems provide an aseptic barrier between the drug vial and syringe, allowing for transfer of fluids without potential contamination from needle punctures or leakage. The double membrane design allows for safe transfers even with vulnerable patient populations like chemotherapy and immunocompromised patients.
For instance, in September 2025, EQUASHIELD made CellSHIELD, a Closed System Transfer Device (CSTD) that keeps healthcare workers and patients safe from dangerous drug exposure. The device makes sure that drugs are handled, prepared, and given out safely, which lowers the risk of contamination. CellSHIELD strengthens EQUASHIELD's position as the best provider of reliable, high-performance CSTDs for hospitals, clinics, and cancer centers.
Closed System Transfer Device Market Insights, By Closing Mechanism - Lead for Secure, Universal Syringe and IV Compatibility
In terms of closing mechanism, the luer lock systems segment is expected to lead the market with largest share in 2025, as they work with standard syringes and IV setups and are safe and leak-proof. They are the best choice for safely moving dangerous drugs, lowering the risk of contamination, and keeping the workflow going because they are easy to use, reliable, and accepted in all healthcare facilities.
For instance, in September 2025, Elcam Medical talks about OLAV™, a closed male Luer connector for handling dangerous drugs. The Luer lock design with automatic closure and locking collar makes sure that connections are safe and leak-free during preparation, transport, and administration. This makes it safer for both healthcare workers and patients and supports closed system transfer workflows.
Closed System Transfer Device Market Insights, By Component - Vial Access Devices Dominate for Safe Hazardous Drug Transfer
In terms of component, the vial access devices segment is expected to hold the largest share of the market in 2025, as they are necessary for safely getting to and moving drugs from vials. They reduce the risk of exposure to dangerous substances, make sure that the right dose is given, and work perfectly with closed system workflows. This makes them essential for safety and compliance in oncology, hospitals, and labs.
For instance, in October 2025, Takeda launched HyHub and HyHub Duo in the U.S. to make it easier to prepare HYQVIA® infusions without using needles. The devices make it easier to move drugs, cut down on the number of steps needed to prepare them, and lower the risks of handling them for both patients and providers. Their design makes them safer, easier to use, and more efficient in both home and clinical infusion settings.
Closed System Transfer Device Market Insights, By Technology - Lead with Reliable, Leak-Free Sealing
In terms of technology, the diaphragm-based devices segment is projected to account for the greatest share of the market in 2025, due to their simple but very effective sealing system makes sure that dangerous drugs do not leak. Given that they work with many different types of vials and syringes, are durable, and have been shown to be reliable in medical settings, they are the most popular technology in hospitals, clinics, and research labs around the world.
Closed System Transfer Device Market Insights, By End Use - Dominate Due To High Chemotherapy and CSTD Use
In terms of end use, the Hospital & Clinics segment is projected to capture the maximum share of the market in 2025, as they perform a great deal of chemotherapy, have strict safety rules, and use CSTD a lot. These places need strong protection for healthcare workers, standard procedures for handling dangerous drugs, and reliable closed systems to keep things clean. This is what drives most of the demand for CSTD in the healthcare sector.
For instance, in February 2025, BD rolled out the PhaSeal Optima Closed System Drug Transfer Device to make drug preparation and administration in hospitals and clinics safer. The system keeps drug vapors from escaping, lowers the risk of contamination, and makes the workflow more efficient by making it easier for healthcare workers to handle chemotherapy and other toxic drugs.
Regional Insights
To learn more about this report, Download Free Sample
North America Closed System Transfer Device Market Analysis & Trends
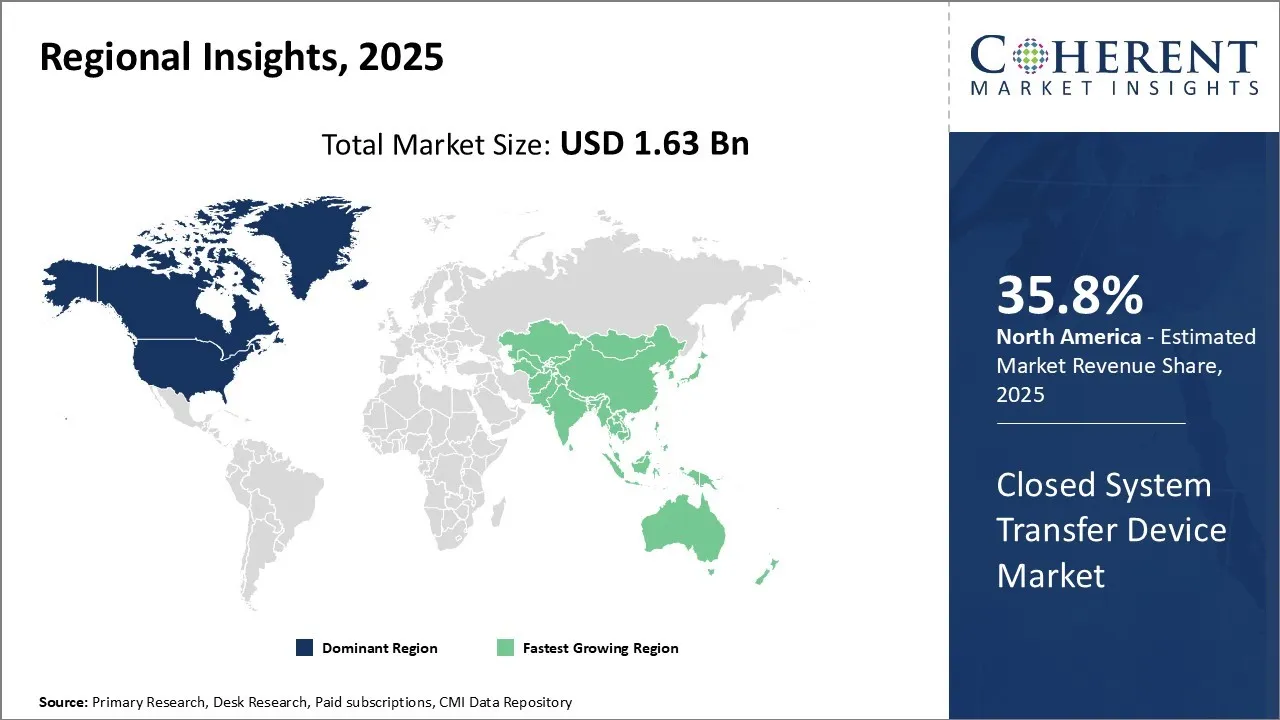
North America has emerged as the dominant region in the closed system transfer device market owing 35.8% in 2025. The region accounts for the largest share owing to stringent regulatory mandates and growing awareness about safe medication practices. The U.S., being the highest spender on healthcare, has implemented strict guidelines through the National Institute for Occupational Safety and Health (NIOSH) to curb needlestick injuries and exposure to hazardous drugs. Most healthcare organizations in the country have adopted closed system drug transfer technologies to comply with the regulatory norms. The presence of leading manufacturers such as Becton, Dickinson and Company along with the availability of numerous generic versions has further boosted product adoption.
Asia Pacific Closed System Transfer Device Market Analysis & Trends
Asia Pacific, on the other hand, is identified as the fastest growing regional market. Factors such as increasing healthcare expenditure, rising incidence of chronic diseases, and growing geriatric population have augmented demand for injectable drugs across hospitals and clinics in the region. Countries like China, India, and Japan are at the forefront of healthcare infrastructure development which is supporting market expansion. Furthermore, shifting focus towards quality medical practices as well as worker safety is encouraging healthcare facilities to incorporate closed drug transfer solutions. While regulations still lag compared to developed markets, growing awareness among healthcare providers regarding needlestick injuries and exposure risks is propelling the Asia Pacific closed system transfer device market upward.
The increasing number of specialty pharmaceutical manufacturing sites and biotechnology hubs in North America have led to a rise in pharmaceutical imports and exports between the region and other parts of the world. This has positively impacted the demand for closed system transfer devices to streamline drug reconstitution processes and uphold global safety and quality standards during transportation. On the pricing front, the availability of cost-effective alternatives from domestic manufacturers in North America and Asia Pacific has rendered the market more affordable for healthcare organizations of all sizes.
Market Report Scope
Closed System Transfer Device Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.63 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 16.7% | 2032 Value Projection: | USD 4.82 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
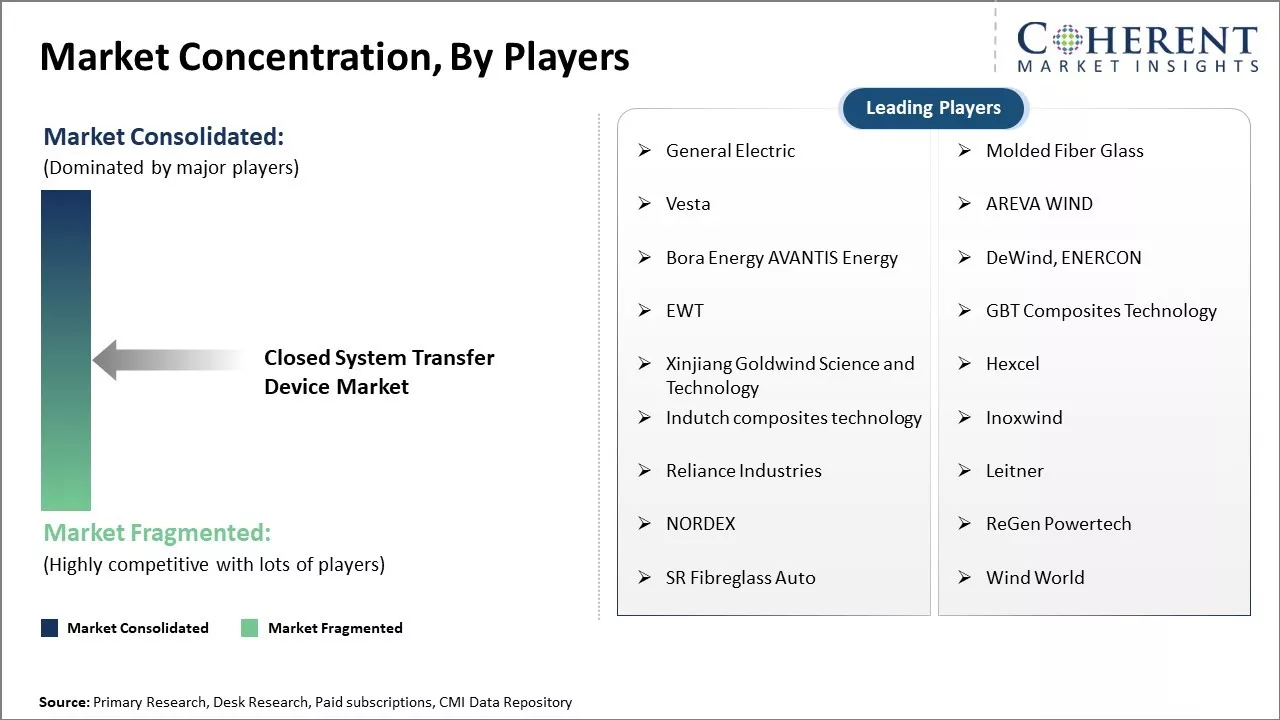
General Electric, Molded Fiber Glass, Vesta, AREVA WIND, Bora Energy AVANTIS Energy, DeWind, ENERCON, EWT, GBT Composites Technology, Xinjiang Goldwind Science and Technology, Hexcel, Indutch composites technology, Inoxwind, Reliance Industries, Leitner, NORDEX, ReGen Powertech, SR Fibreglass Auto, and Wind World |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Closed System Transfer Device Market Drivers
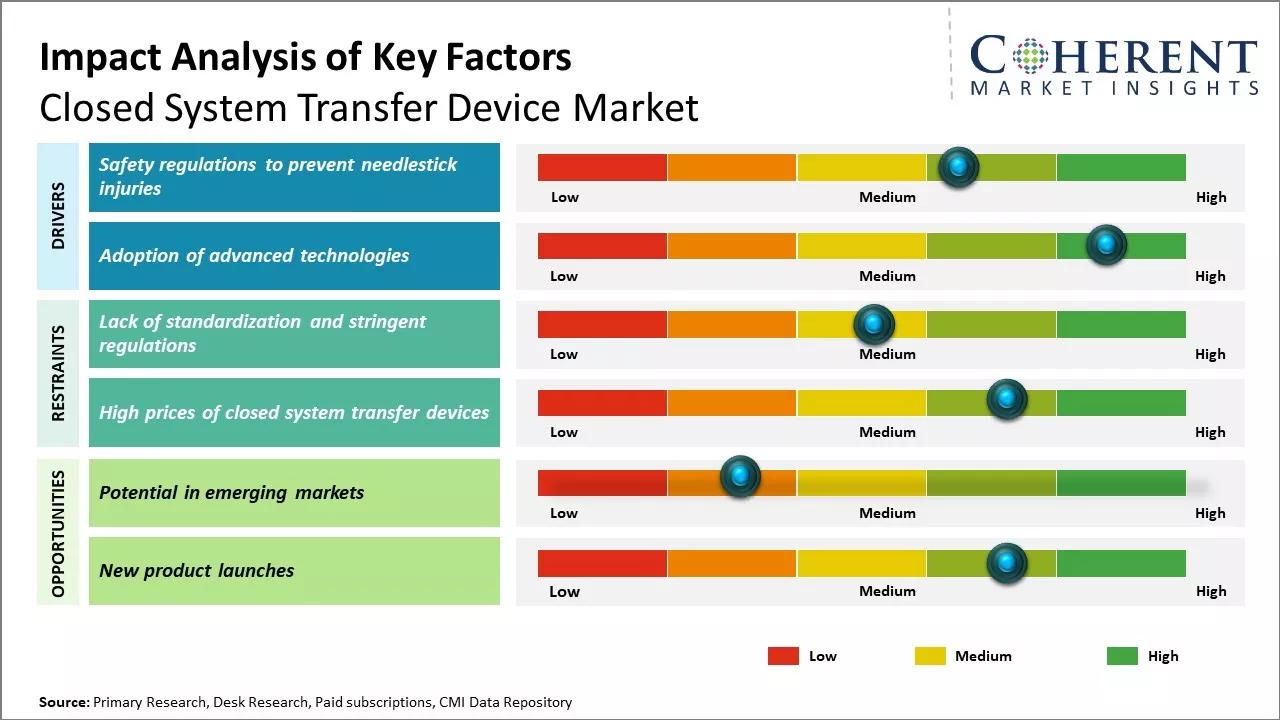
Safety regulations to prevent needlestick injuries
With the growing risk of infections associated with needlestick injuries, major regulatory bodies have introduced stringent safety norms and guidelines to curb this hazard in healthcare settings. The Centers for Disease Control and Prevention (CDC) in the U.S. has issued mandatory sharps injury prevention regulations via the Needlestick Safety and Prevention Act to protect healthcare workers from accidental needlestick injuries and exposure to bloodborne pathogens. Likewise, the European Union has rolled out several directives focusing on safe handling of sharps and injection devices.
The implementation of these regulations has posed immense pressure on healthcare facilities to phase out the use of conventional syringes and vials that may lead to needlestick injuries during the reconstitution or administration of drugs. This has boosted the demand for advanced closed drug transfer systems that enable contained operations and zero exposure risk. Unlike conventional open vial systems, closed drug transfer devices ensure complete protection against accidental pricks by encapsulating the needle or cannula and isolating the drug product from the external environment. The focus on safer handling of hazardous drugs like antineoplastic injectables has further stimulated the adoption of these engineered safety solutions across hospitals, clinics, and other healthcare settings.
Market Concentration and Competitive Landscape
To learn more about this report, Download Free Sample
Adoption of advanced technologies
Tremendous advancements in drug delivery technologies that enhance patient safety, therapeutic efficacy, and optimize workflows have been witnessed. Closed drug transfer systems are pioneering technologies that leverage engineering prowess to address one of the most pressing issues concerning hazardous drug exposures faced by healthcare professionals worldwide. Beyond fulfilling safety compliance requirements, these systems allow for more efficient large scale compounding operations as they automate transfer and eliminate spillage risks. Their seamless integration with robotic infusion technology and workflow automation modules have enabled next-generation pharmacy compounding centers to achieve higher throughput while guaranteeing sterility.
Growing oncology drug pipelines have catalyzed the demand for specialized injectable preparations in healthcare facilities. At the same time, rapid technological evolution has equipped clinical staff with more potent and precise options for administering cancer therapeutics. In this scenario, closed drug transfer equipment has emerged as an indispensable tool supporting safe and reliable reconstitution of antineoplastic drugs. Their sophisticated mechanical designs combining hermetically sealed enclosures, automated cannula shielding mechanisms, and fluid handling modules offer a robust platform for advanced compounding needs. As novel biologic therapies enter the scene, healthcare providers are willing to strengthen workflow safety with cutting-edge closed drug transfer solutions.
Closed System Transfer Device Market Opportunities
Potential in emerging markets
As regulations tighten around hazardous drug handling, the demand will continue growing. Larger hospitals and oncology centers in particular represent a sizable addressable market. Innovation in closed system designs may help reduce costs and complexity of use. Increasing compatibility of closed systems with existing medical equipment expands applicability. Rising awareness about occupational exposure risks also heightens the appeal of closed systems' safety benefits among hospitals and staff.
Analyst Opinion (Expert Opinion)
- The global Closed System Transfer Device market is growing rapidly because a growing number of healthcare settings are using them, new technologies are being developed, and strict rules are in place. According to industry data, revenue has been steadily rising, which is a sign of growing demand for CSTDs in hospitals, oncology clinics, and outpatient facilities.
- Segment analysis shows that membrane-to-membrane systems are the most popular as their dual-barrier design reduces the risk of contamination. Needleless systems are also popular because they are easier to use and safer for sharps. Luer-lock and push-to-turn systems are still the most common ways to close things, but newer systems are becoming more popular because they are easier to use. Vial access devices and syringe safety devices are two components that drive a lot of market demand, which is in line with the high volume of drug preparation activities.
- North America is the region that has adopted the most, due to its advanced healthcare infrastructure and strict enforcement of rules. Asia Pacific, on the other hand, is the region that is growing the fastest due to more healthcare investments and more awareness of workplace dangers.
- Some of the main reasons are stricter safety rules, new technology for containment and automated compounding, and more awareness of workplace safety. Some of the problems are that drug delivery systems don't always work together and that prices are high in areas where people are sensitive to them.
Closed System Transfer Device Industry News
- In October 2023, EQUASHIELD LLC. (US) is a company that specializes in providing closed system transfer devices (CSTDs) for the safe handling of hazardous drugs in healthcare settings. Syringe Unit received additional Food and Drug Association approval for full-volume use, providing a cost-effective solution for medication compounding & administering needs
Market Segmentation
- Type Insights (Revenue, USD Bn, 2025 - 2032)
- Needless Systems
- Membrane to Membrane Systems
- Closing Mechanism Insights (Revenue, USD Bn, 2025 - 2032)
- Colour to Colour Alignment Systems
- Lueker Lock Systems
- Push to Turn systems
- Click to Lock Systems
- Component Insights (Revenue, USD Bn, 2025 - 2032)
- Syringe Safety Devices
- Vial Access Devices
- Bag Access Devices
- Other
- Technology Insights (Revenue, USD Bn, 2025 - 2032)
- Compartmentalize Devices
- Diaphragm Based Devices
- Air Filtration Devices
- End Use Insights (Revenue, USD Bn, 2025 - 2032)
- Hospitals & Clinics
- Ambulatory Surgery Centres
- Research Laboratories
- Others
- Regional Insights (Revenue, USD Bn, 2025 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East & Africa
- GCC Countries
- South Africa
- Rest of Middle East & Africa
- Key Players
- General Electric
- Molded Fiber Glass
- Vesta
- AREVA WIND
- Bora Energy AVANTIS Energy
- DeWind, ENERCON
- EWT
- GBT Composites Technology
- Xinjiang Goldwind Science and Technology
- Hexcel
- Indutch composites technology
- Inoxwind
- Reliance Industries
- Leitner
- NORDEX
- ReGen Powertech
Sources
Primary Research Interviews
- Pharmaceutical & Biotech Companies
- Medical Device Manufacturers
- Closed System Transfer Device (CSTD) Suppliers
- Hospital & Oncology Pharmacists
- Healthcare Safety Consultants
- Others
Databases
- Bloomberg Terminal
- Thomson Reuters Eikon
- Others
Magazines
- Pharmaceutical Technology Magazine
- Medical Device & Diagnostic Industry (MD+DI)
- Oncology Times
- Chemical Engineering Magazine
- Others
Journals
- Journal of Oncology Pharmacy Practice
- International Journal of Pharmaceutics
- American Journal of Health-System Pharmacy
- Medical Devices: Evidence and Research
- Others
Newspapers
- Financial Times
- The Wall Street Journal
- Reuters
- Bloomberg News
- Others
Associations
- ISOPP – International Society of Oncology Pharmacy Practitioners
- USP – United States Pharmacopeia
- European Society of Oncology Pharmacy (ESOP)
- American Society of Health-System Pharmacists (ASHP)
- Others
Public Domain Sources
- U.S. Food and Drug Administration (FDA)
- World Health Organization (WHO)
- European Medicines Agency (EMA)
- Centers for Disease Control and Prevention (CDC)
- National Institute for Occupational Safety and Health (NIOSH)
- Others
Proprietary Elements
- CMI Data Analytics Tool
- Proprietary CMI Existing Repository of Information for the Last 8 Years
*Definition: The closed system transfer device market provides products that minimize the risk of environmental contamination and exposure during the transferring of hazardous drugs. These closed system drug transfer devices provide either mechanical or mechanical with air filtration systems to transfer liquids between containers while preventing the escape of toxic vapor particles into the surrounding environment. They are commonly used in hospitals and oncology clinics when preparing and administering chemotherapy drugs and other hazardous medications to improve workplace safety.
Share
Share
About Author
Nikhilesh Ravindra Patel is a Senior Consultant with over 8 years of consulting experience. He excels in market estimations, market insights, and identifying trends and opportunities. His deep understanding of the market dynamics and ability to pinpoint growth areas make him an invaluable asset in guiding clients toward informed business decisions. He plays a instrumental role in providing market intelligence, business intelligence, and competitive intelligence services through the reports.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients