Clinical Trial Biorepository And Archiving Solutions Market Size and Trends
Global clinical trial biorepository and archiving solutions market is estimated to be valued at USD 4.98 Bn in 2025 and is expected to reach USD 9.96 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 10.4% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
Increasing R&D expenditure by biopharmaceutical companies and growing adoption of precision medicine boosts demand for effective clinical trial biorepository solutions. Furthermore, rising prevalence of various chronic diseases and growth in clinical trial activities can drive the market growth. Various initiatives by market players to expand their global footprint through strategic partnerships can drive the market growth. However, lack of harmonization in regulations and standardization practices can hamper the clinical trial biorepository and archiving solutions market growth during the forecast period.
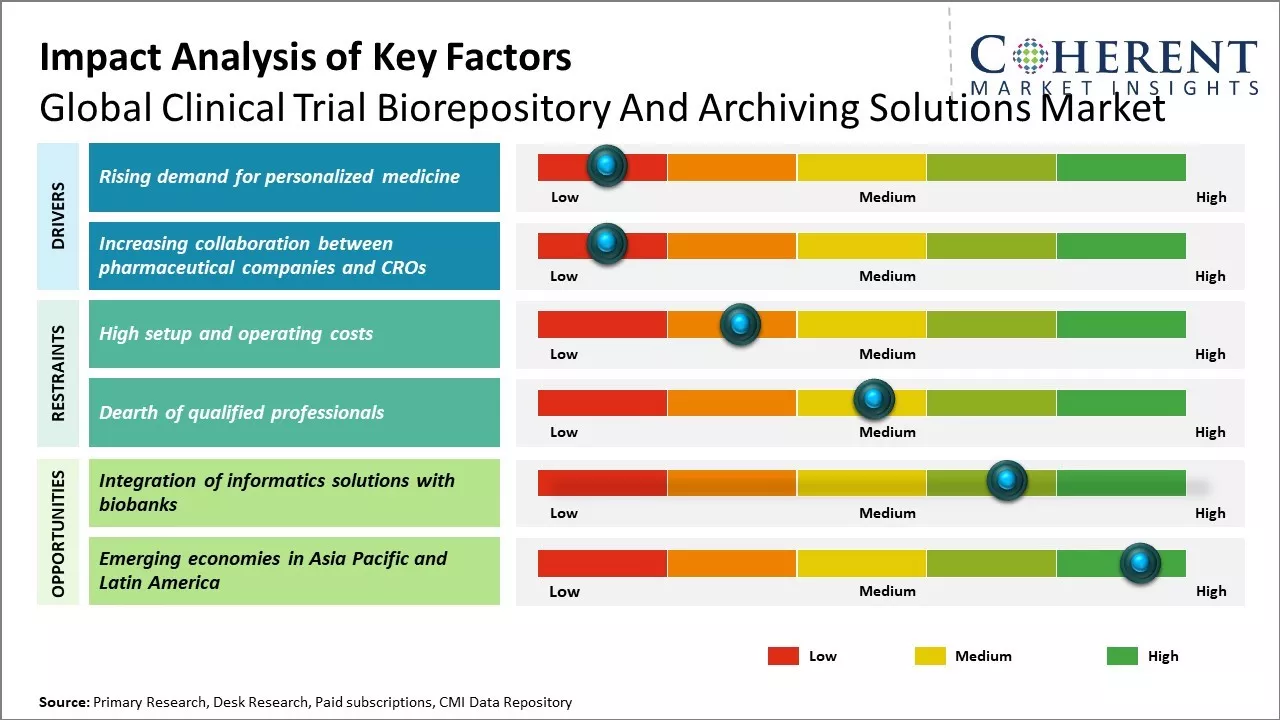
Rising demand for personalized medicine
As scientific advances continue to revolutionize healthcare, personalized medicine has become an important driver in clinical research. Personalized medicine focuses on tailoring treatments and prevention strategies based on a patient's genetic profile and other biological factors. This requires extensive molecular profiling and genomic analysis of clinical samples to better understand how a person's unique genetic profile impacts disease risk, progression and response to therapy. Thus, biorepositories play a key role in facilitating personalized medicine research by securely storing and archiving biological specimens and associated clinical datasets that can be used to identify biomarkers and develop targeted therapies. Their role has become integral to furthering research in precision medicine by enabling scientists to examine large numbers of biological samples in clinical studies focused on individualizing patient care. Growing emphasis on personalized healthcare boosts demand for reliable solutions that can effectively manage the massive amounts of sensitive data and specimens being generated through personalized medicine research efforts. In response to escalating challenges of chronic diseases and growing need for personalized treatments, AI-driven precision medicine is increasingly viewed as a transformative solution in healthcare. For instance, in May 2025, latest blueprint from Info-Tech Research Group, titled "AI-Powered Precision Medicine for Improved Patient Outcomes," offers healthcare organizations a strategic roadmap to effectively leverage AI technologies. By integrating AI into diagnostic and therapeutic practices, healthcare providers can enhance treatment precision, optimize patient outcomes, and streamline healthcare delivery. Moreover, In October 2022, GSK plc and Tempus, a precision medicine company based in the U.S., signed a three-year collaboration agreement. This agreement granted GSK plc access to Tempus' AI-enabled platform that includes a library of de-identified patient data.
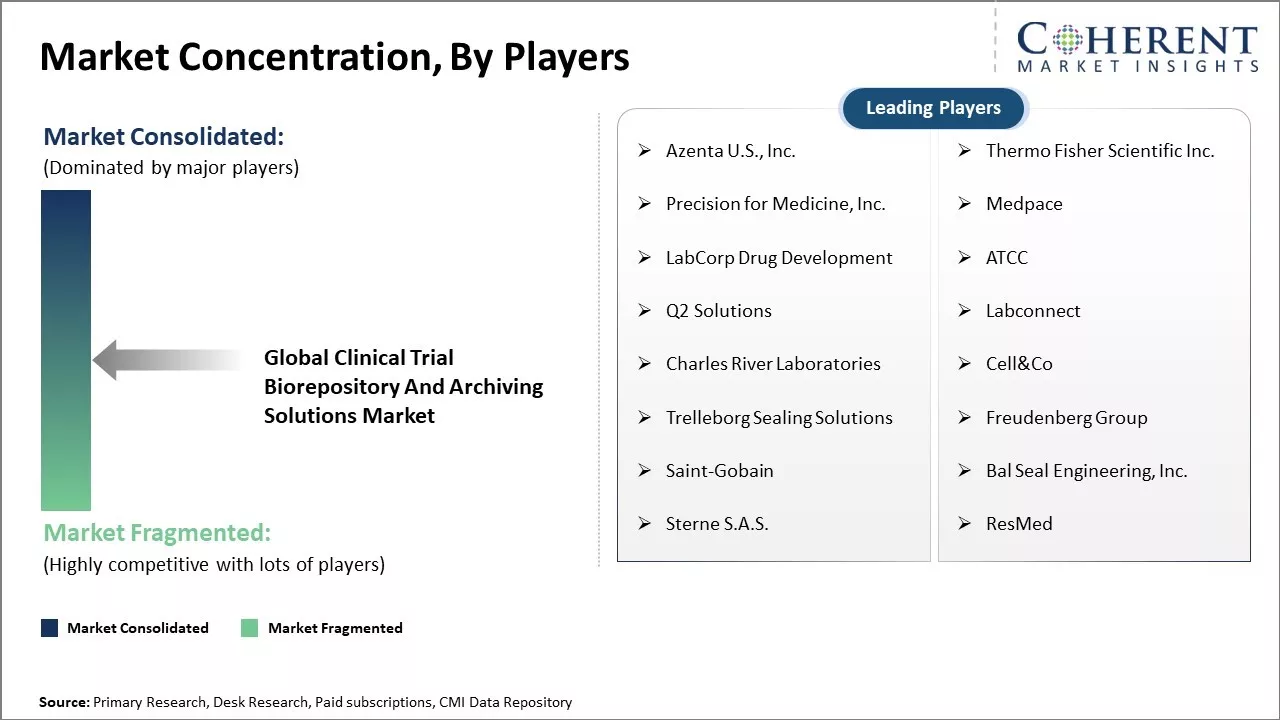
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Increasing collaboration between pharmaceutical companies and CROs
Increasing partnerships and collaborations between pharmaceutical companies and contract research organizations for clinical trial projects can drive the market growth. Large pharmaceutical firms outsource clinical trial activities to CROs as these specialists can offer advantages like reduced costs, faster timelines, and specialized expertise that drug makers may lack internally. CROs expands their service offerings to provide end-to-end support for trials. This includes assisting with newer areas like real-world evidence research, site feasibility assessments, and genomic/companion diagnostic development. As pharmaceutical-CRO relationships strengthen, it boosts need for integrated data and sample management solutions that can support collaborative, multi-site research. Centralized clinical trial biorepositories have become an important platform enabling seamless data and sample sharing between sponsor companies, CRO partners as well as clinical investigators. Their role in facilitating collaboration across research networks can boost demand for sophisticated archiving solutions that comply with data privacy regulations and enable partners to efficiently work together on trials.
Key Takeaways from Analyst:
Global clinical trial biorepository and archiving solutions market can witness growth in the near future. North America currently dominates the market due to stringent regulations and high R&D expenditure. Asia Pacific region is expected to witness the fastest growth, owing to increasing outsourcing of clinical trials to countries like China and India.
Rising R&D investment in drug development by biopharma companies and growing involvement of CROs in clinical trials can drive the market growth. Furthermore, increasing prevalence of diseases like cancer prompt more clinical trials, thus, boosting demand for biorepository and archiving solutions. Growing orphan drug development can offer market growth opportunities.
High setup and maintenance costs of biorepositories can hamper the market growth. Lack of harmonization in regulatory guidelines across regions can hamper the market growth.
Market Challenges: High setup and operating costs
High setup and operating costs can hamper the global clinical trial biorepository and archiving solutions market growth. Establishing and maintaining a biobank requires huge capital investments for setting up infrastructures such as storage facilities that meet regulatory standards for temperature-controlled storage of samples. Advanced equipment such as automated sample storage systems and barcode readers that enable efficient management and tracking of thousands of samples can contribute significantly to initial costs. Once set up, operational costs such as maintaining stringent compliance with quality management standards, training and certifying staff to handle sensitive samples, ongoing maintenance of facilities and equipment also impose substantial financial burdens on biobanks over time. Significant costs are also involved in record keeping, sample labelling, and retrieval along with maintenance of sample integrity during long term archival. The need to replace aging storage equipment on a periodic basis further increases the operating expenses. For biobanks associated with clinical trial sites in remote or less developed areas, transportation and logistics challenges also boost distribution costs of moving samples to and from centralized facilities.
Market Opportunities: Integration of informatics solutions with biobanks
Integration of informatics solutions with biobanks can provide significant opportunities for global clinical trial biorepository and archiving solutions market growth. Biobanks house vast amounts of biological samples and associated health data from patients. However, without proper informatics systems, it can be challenging to manage, track and analyze these resources efficiently. Integrating biobank management systems with advanced informatics tools allows samples and data to be securely stored, easily searched, linked and shared for research purposes. This enables researchers to gain novel insights by studying large volumes of correlated biological and health information. With continuous advancements in fields like cloud computing, artificial intelligence and blockchain technologies, informatics solutions are becoming more powerful, scalable and collaborative in nature. Biobanks can leverage these technologies to improve workflows, address challenges like data integration and standardization, and accelerate the drug discovery process. For example, AI and machine learning algorithms applied on biobank data resources can help identify new biomarkers and therapeutic targets. Blockchain integration provides transparency of sample and data usage along with enhanced security. These solutions enhance the value of biobanked resources, addressing the growing demand from pharmaceutical companies for real-world evidence in clinical trials.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
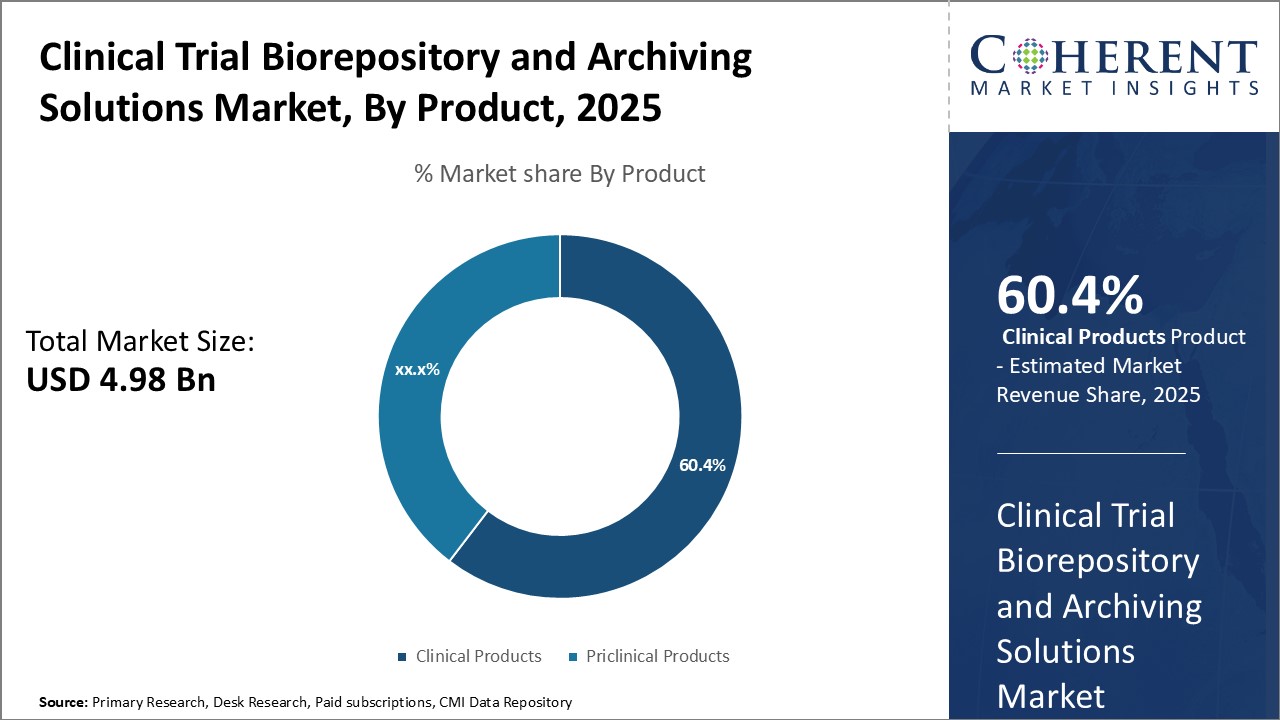
By Product - The importance of patient outcomes:
In terms of product, clinical products segment is estimated to contribute the highest market share of 60.4% in 2025, as hospitals and healthcare providers increasingly prioritizing patient care and outcomes. Clinical products play a vital role in streamlining complex trial workflows, facilitating regulatory compliance, and ensuring samples are safely stored and accessible to researchers. This allows practitioners to deliver cost-effective treatments tailored to individual patients, thus, boosting demand for solutions that optimize clinical research processes.
By Phase - Stringent regulatory oversight boosts Phase III demand
In terms of phase, phase III segment is estimated to contribute the highest market share of 30.5% in 2025, due to rigorous regulatory standards governing late-stage trials. As Phase III trials involve large patient populations and aim to confirm drug efficacy and safety, non-compliance with protocols can delay drug approvals or result in costly product recalls. Biorepositories and archiving solutions help sponsors and sites to comply with international standards through automated sample logging, real-time inventory tracking, and audit-ready chain of custody records. This provides regulators confidence in data integrity and human subject protection, thus, driving Phase III segment growth.
By Services - Emphasis on full-service offerings bolsters biorepository leadership
In terms of services, biorepository services segment is estimated to contribute the highest market share of 51.71% in 2025, as healthcare stakeholders increasingly value streamlined solutions. Integrated biorepositories offering sample receipt, storage, retrieval and disposal help research teams to stay focused on critical tasks like patient recruitment and trial design. Full-service providers also spare sponsors the risks and expenses associated with in-house management. Their expertise in regulatory compliance and sample security thereby meets a key priority for stakeholders and strengthens biorepository segment dominance.
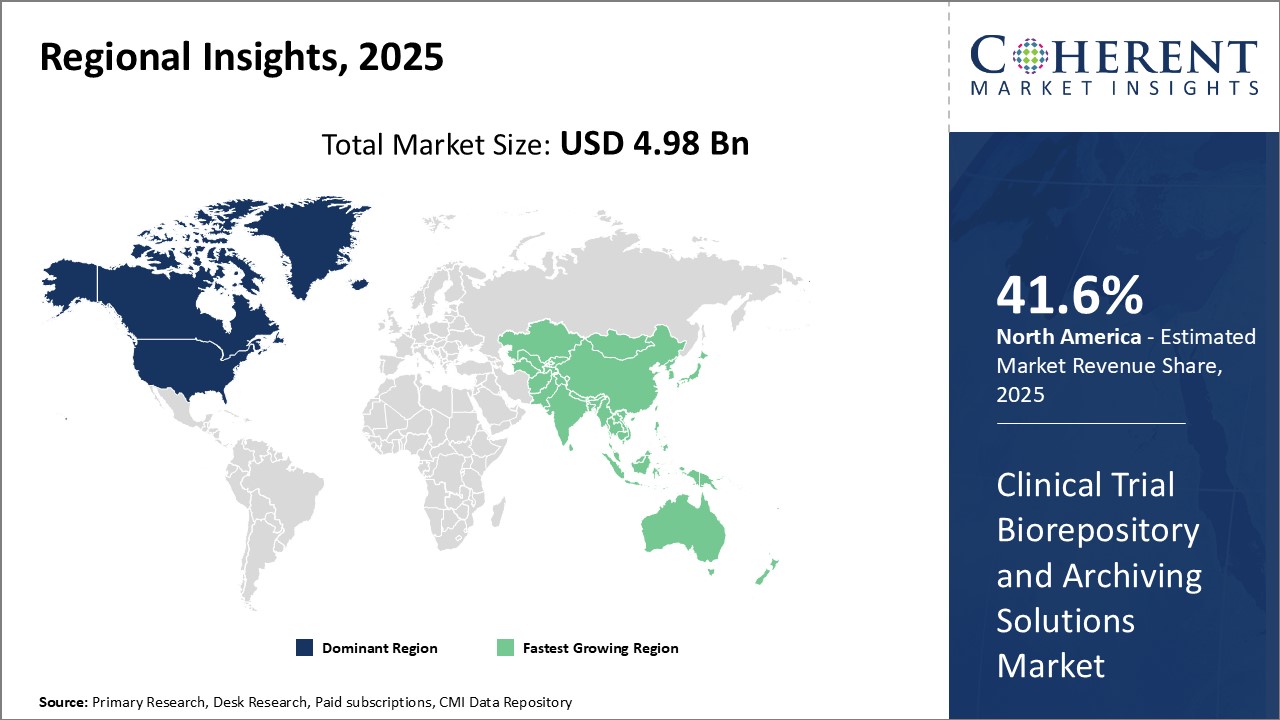
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America currently dominates the global clinical trial biorepository and archiving solutions market, with an estimated market share of 41.6% in 2025 owing to heavy investments in clinical trials by pharmaceutical and biotech companies based in the U.S. and Canada. With the presence of top clinical research organizations, contract research organizations and CROs, North America has established itself as the leader in clinical trials conducted globally each year. According to a recent industry report, the U.S. accounted for over 50.5% of market share in 2021 due to well-established medical and academic institutions that conduct clinical research. Furthermore, presence of major market players such as Brooks Life Sciences, Tecan Trading AG and Hamilton Company in the region boosts demand for state-of-the-art biorepository solutions.
Asia Pacific has emerged as the fastest growing regional market for Clinical Trial Biorepository And Archiving Solutions over the past decade. Countries such as China, India, Japan and South Korea have witness immense development of their pharmaceutical, biologics and chemicals sectors.
Market Report Scope
Clinical Trial Biorepository and Archiving Solutions Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 4.98 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 10.4% | 2032 Value Projection: | USD 9.96 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Azenta U.S., Inc., Thermo Fisher Scientific Inc., Precision for Medicine, Inc., Medpace, LabCorp Drug Development, ATCC, Q2 Solutions, Labconnect, Charles River Laboratories, Cell&Co, Trelleborg Sealing Solutions, Freudenberg Group, Saint-Gobain, Bal Seal Engineering, Inc., Sterne S.A.S., ResMed |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Clinical Trial Biorepository and Archiving Solutions Industry News
- In April 2024 The Lupus Nexus program was developed by The Lupus Research Alliance (LRA) and Lupus Therapeutics (LT) in partnership with industry partners. To speed up lupus research, this incorporates a novel biorepository, lupus registry, and data exchange platform. The Lupus Landmark research (LLS), five-year longitudinal research involving 3,500 lupus patients, is at the heart of the effort. The Data Repository, Exchange, and Analysis (DREAM) platform will be able to receive biospecimens and a wealth of clinical and patient-reported data from the LLS to aid in ongoing lupus research efforts.
- In October 2021, Azenta Life Sciences, a provider of life sciences services including genomics, cryogenic storage, automation, and informatics, and Cleveland Clinic inaugurated a new 22,000-square-foot facility for biospecimen sample management and repository on the Cleveland Clinic’s main campus. This facility aims to significantly enhance Cleveland Clinic's biobanking capabilities and accelerate translational research efforts.
*Definition: Global Clinical Trial Biorepository and Archiving Solutions Market provides products and services to support pharmaceutical and biotechnology companies in storing, managing, and shipping clinical trial samples. This includes hardware like freezers and storage systems, software for sample inventory management and chain of custody tracking, as well as laboratory and support services for processing, accessioning, and retrieving samples in compliance with regulatory standards. These integrated solutions help clinical trial sponsors properly manage biological samples collected during drug development research to advance new drug discoveries and validate clinical results.
Market Segmentation
- Product Insights (Revenue, USD Bn, 2020 - 2032)
- Preclinical Products
- Clinical Products
- Phase Insights (Revenue, USD Bn, 2020 - 2032)
- Phase I
- Phase II
- Phase III
- Phase IV
- Services Insights (Revenue, USD Bn, 2020 - 2032)
- Biorepository Services
- Warehousing & Storage
- Transportation
- Sample Processing
- Others
- Archiving Solution Services
- Database Indexing and Management
- Scanning & Destruction
- Biorepository Services
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Azenta U.S., Inc.
- Thermo Fisher Scientific Inc.
- Precision for Medicine, Inc.
- Medpace
- LabCorp Drug Development
- ATCC
- Q2 Solutions
- Labconnect
- Charles River Laboratories
- Cell&Co
- Trelleborg Sealing Solutions
- Freudenberg Group
- Saint-Gobain
- Bal Seal Engineering, Inc.
- Sterne S.A.S.
- ResMed
Share
Share
About Author
Manisha Vibhute is a consultant with over 5 years of experience in market research and consulting. With a strong understanding of market dynamics, Manisha assists clients in developing effective market access strategies. She helps medical device companies navigate pricing, reimbursement, and regulatory pathways to ensure successful product launches.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
