Cellular Health Screening Test Market is estimated to be valued at USD 3.67 Bn in 2025 and is expected to reach USD 8.37 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 12.5% from 2025 to 2032.
The cellular health screening test market has strong potential for growth driven by rising awareness about preventive healthcare and early disease detection. As lifestyles become increasingly sedentary and rates of chronic diseases rise, the demand for non-invasive screening options will increase. Furthermore, recent technological advances have made tests more accurate and accessible, encouraging broader adoption. Younger demographics are increasingly open to routine screening as a way to stay on top of their health.
However, limited insurance coverage for such tests and their cost remains a key restraint in many markets. Approval challenges and varying regulations across regions also impact market expansion. While North America and Western Europe currently dominate due to regulatory clarity and reimbursement policies, Asia Pacific is expected to emerge as the fastest growing regional market. Rising incomes and demand for quality healthcare present long-term opportunities for market participants.
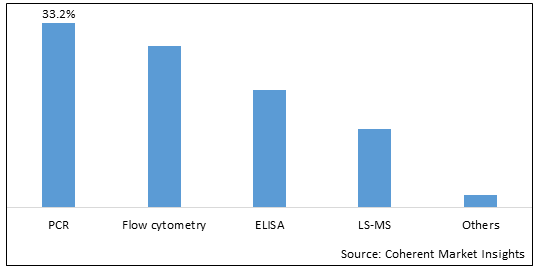
The cellular health screening test market is segmented based on test type, sample type, product type, technology, end user, and region. Based on technology, the market is segmented into PCR, Flow cytometry, ELISA, LC-MS, and others. PCR (Polymerase chain reaction) is dominating the market as it is gaining popularity due to continued technological advancements
Global Cellular Health Screening Test Market Regional Insights:
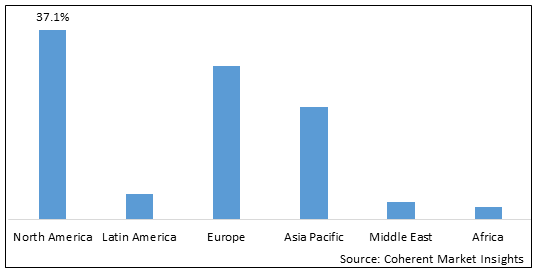
- North America is estimated to be the largest market for cellular health screening test during the forecast period, which accounted for over 37.1% of the market share in 2025. The growth of the market in North America is attributed to the high adoption of preventive diagnostic tests, presence of key players, and increasing research on cellular ageing.
- Europe is expected to be the second-largest market for cellular health screening test, which accounted for over 30.1% of the market share in 2025. The growth of the market is attributed to the rising prevalence of chronic diseases, increasing healthcare expenditure, and growing awareness regarding cellular health screening.
- Asia Pacific is expected to be the fastest-growing market for cellular health screening test, which is expected to grow at a CAGR of over 22.1% during the forecast period. The growth of the market in Asia Pacific is attributed to the expanding patient pool, improving healthcare infrastructure, and favorable government initiatives.
Figure 1. Global Cellular Health Screening Test Market Share (%), by Region, 2025
To learn more about this report, Download Free Sample
Global Cellular Health Screening Test Market Drivers:
- Rising Prevalence of Chronic Diseases: The rising prevalence of chronic diseases such as cancer, cardiovascular diseases, and diabetes is a major factor driving the growth of the cellular health screening test market. Cellular health screening tests can detect biochemical imbalances at early stages, enabling timely treatment and management of chronic diseases. For instance, according to data published in John Wiley & Sons, Inc, a U.S.-based multinational publishing company, in January 2023, 1,958,310 new cancer cases and 609,820 cancer deaths are projected to occur in the U.S. in 2023.
- Advancements in Cellular Analysis Technologies: Technological advancements in cellular analysis techniques have enabled more efficient and accurate measurement of biomarkers of cellular health. Advanced cellular analysis technologies, such as PCR, LC-MS, flow cytometry, and next-generation sequencing, have expanded the range of biomarkers that can be tested through cellular screening. Moreover, automation in lab testing has increased the throughput for cellular screening. Furthermore, innovations in cellular analysis hardware, reagents, and software will support the adoption of cellular testing in clinical applications.
- Growing Adoption of Personalized Medicine: The shift towards precision medicine and preventive healthcare is driving the uptake of cellular screening. Cellular screening provides personalized insights based on an individual's biochemical and genetic profile. For instance, individuals with shorter telomeres or high oxidative stress can be recommended targeted lifestyle or dietary changes for improving cellular health. Precision medicine relies heavily on advanced diagnostic methods. Increasing adoption of personalized medicine approaches in clinical settings is expected to propel the use of cellular screening.
- Expanding Applications in Health Monitoring: Cellular screening tests are gaining traction for regular health monitoring and tracking of specific therapies. Changes in biomarkers over time can indicate improving or worsening biochemical balance. Cellular tests are increasingly being combined with wearable devices to provide insights through continuous health monitoring. For example, repeat testing of oxidative stress biomarkers can demonstrate the impact of an antioxidant supplementation regimen. The growing use of cellular screening in health monitoring and tracking therapeutic response will support market expansion.
- Development of Testing Services for Health Tracking: Cellular screening tests can be incorporated by healthcare providers into routine health tracking services for patients during annual physicals or regular checkups. Changes in biomarkers over time would allow better monitoring of any biochemical imbalances. For instance, a drop in vitamin D levels across periodic testing can prompt earlier nutrition interventions before deficiencies worsen. Providers can also offer packages or subscriptions for regular cellular screening tailored to individual health needs.
Global Cellular Health Screening Test Market Opportunities
- Direct-to-Consumer Testing Services: Direct-to-consumer models for cellular screening diagnostics provides opportunities for market growth by increasing consumer access to testing. Several companies now offer telomere length testing, nutrient biomarker testing, etc. directly to consumers without the need for prior physician approval. This provides consumers convenient access to cellular health monitoring tools for lifestyle advice and disease prevention. Partnerships with wearable device companies can further drive the adoption of direct-to-consumer cellular screening.
- Advancements in Cellular Analysis Technologies: Technological advancements in cellular analysis techniques have enabled more efficient and accurate measurement of biomarkers of cellular health. Advanced cellular analysis technologies, such as PCR, LC-MS, flow cytometry, and next-generation sequencing, have expanded the range of biomarkers that can be tested through cellular screening. Moreover, automation in lab testing has increased the throughput for cellular screening. Furthermore, market players are focusing on the launch of technologically advanced new products, which is expected to propel the market growth over the forecast period. For instance, in July 2022, Bloom Diagnostics, a fully-featured laboratory management solution for hospitals and commercial laboratories, announced the launch of the Bloom Inflammation Test, which is designed to measure and detect the C-Reactive Protein (CRP) in the bloodstream. The test is designed to be used by professional clinicians, allowing doctors and pharmacists to rapidly measure and quantify inflammation in anyone aged 18 and above.
- Testing Services for Clinical Trials: Cellular screening is being increasingly utilized to monitor biochemical indicators related to disease progression, therapeutic response, and health outcomes during clinical trials and research studies. Cellular biomarkers enable the evaluation of subtle biochemical effects and changes for experimental drugs, supplements, or interventions. Clinical researchers anticipate broader applications as more cellular biomarkers are validated. Testing services tailored for clinical trials present an excellent growth opportunity.
- Partnerships with Healthcare Providers: Diagnostics companies offering cellular screening can drive adoption by partnering with large healthcare networks and regional providers. Offering co-branded or customized testing services, sample collection arrangements, integrated reporting, and discounts on multi-test packages will incentivize providers to recommend cellular screening to patients. These strategic partnerships will be key to position cellular screening in routine standard of care and expand market reach.
Cellular Health Screening Test Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 3.67 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 12.5% | 2032 Value Projection: | USD 8.37 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Genova Diagnostics, Cell Science Systems, Life Length, Repeat Diagnostics, Titianovo, DNA Labs India, Segterra, Telomere Diagnostics, Spectracell Laboratories, Zimetry LLC, Immundiagnostik AG, Cleveland HeartLab, Inc., DNA Diagnostics Center, Immuno Diagnostics, Cell Science Systems Corporation, Life Length S.L., Repeat Diagnostics Inc., SpectraCell Laboratories, Telomere Diagnostics, Bloom Diagnostics, Kindbody, GRAIL, Inc, Atomo Diagnostics, OmegaQuant, Allara, and Câ‚‚N Diagnostics |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Global Cellular Health Screening Test Market Trends:
- Increasing Investments in Cellular Ageing Research: Cellular ageing and biomarkers related to longevity are a key research focus in the healthcare industry. Several companies and research institutes are actively investigating associations between biochemical biomarkers like telomere length, epigenetic changes, senescent cell accumulation, and human healthspan. An aging population also underscores the need for advancing cellular ageing science. The insights from this emerging research are expected to bolster the adoption of cellular tests that assess biological age.
- Development of Integrated Multi-analyte Assays: Diagnostics companies are developing integrated assays that combine markers of oxidative stress, inflammation, hormones, vitamins, and cellular aging. Multi-analyte panels provide a more comprehensive snapshot of overall cellular health compared to individual biomarker tests. For instance, In May 2022, Câ‚‚N Diagnostics, a leader in advanced brain health diagnostics that offers the PrecivityAD blood test as an aid to Alzheimer’s disease diagnosis, announced its latest innovative offering for enhancing care in brain health: a high-resolution mass spectrometry-based plasma tau multi-analyte assay (p-tau MAA) for Research Use Only (RUO). This assay uses a small sample of blood to precisely and simultaneously measure different phosphorylated and nonphosphorylated forms of the tau protein including forms with phosphorylation at the tau217 and tau181 sites
- Partnerships between Diagnostics and Pharmaceutical Companies: Collaborations between diagnostics developers and pharmaceutical companies are on the rise to co-develop companion diagnostic tests for drugs under clinical evaluation. Cellular screening tests can potentially serve as companion diagnostics to identify patient subgroups most likely to benefit from certain therapies. For instance, an oxidative stress biomarker test can aid in testing drugs that target cellular oxidative damage pathways. These strategic partnerships will support the utility of cellular screening.
- Growing Industry Focus on Liquid Biopsy Testing: Liquid biopsy tests that analyze biomarkers in blood, urine, or saliva are gaining significant market focus driven by their non-invasive nature. Cellular screening tests perfectly align with the liquid biopsy theme by measuring multiple biochemical markers from such samples. Leading diagnostics companies are actively expanding their liquid biopsy product portfolios. The liquid biopsy trend will further propel research and funding for cellular biomarker assays.
Global Cellular Health Screening Test Market Restraints:
- Reimbursement Challenges for Cellular Screening Tests: Lack of favorable reimbursement for newer advanced diagnostics has constrained the market growth for cellular screening tests. Out-of-pocket payment requirements can impact consumer adoption in the absence of insurance coverage or Medicare payment approval. Companies lobbying for positive reimbursement decisions for emerging molecular diagnostics tests face an uphill battle. Reimbursement challenges will hamper the realization of the full commercial potential.
- High Costs and Complexity of Cellular Analysis Platforms: Many core lab analysis platforms used for measuring key cellular biomarkers involve high instrument and reagent costs. Flow cytometers, PCR systems, and high-end mass spec systems cost several thousands of dollars in capital investment. The need for trained personnel further adds to costs for healthcare organizations. Smaller clinical labs may be deterred from offering cellular screening due to high costs, limiting wider adoption.
Counter balance: Manufacturers should miniaturizing cellular analysis platforms and using microfluidic systems can reduce the amount of reagents and cells needed for experiments, which can lower costs.
- Lack of Consistent Clinical Validation for Cellular Biomarkers: While research continues, cellular biomarkers like telomere length, oxidative stress, etc. lack consistent validation about their direct clinical utility in disease diagnosis or monitoring. Conflicting study results on the predictive value of some newer cellular biomarkers has curtailed their incorporation into recommended clinical screening guidelines. Lack of sufficient evidence-based clinical validation slows the mainstream adoption of certain cellular screening tests.
Figure 2. Global Cellular Health Screening Test Market Share (%), by Product Type, 2025
To learn more about this report, Download Free Sample
Key Developments:
Product Launches:
- In January 2021, Kindbody, a leading fertility and family-building care company, announced the launch of Kind at Home, its consumer products division to support people across their entire reproductive journey, beginning with simple-to-use at-home fertility hormone tests for both women and men. The Kind at Home fertility test kit provides consumers comprehensive information about their fertility status from the convenience of home with personalized recommendations by Kindbody physicians that can be carried to the patient's virtual or in-person clinic appointment.
- In November 2021, Allara is a developer of a virtual care platform designed for women struggling with complex, chronic health conditions, starting with polycystic ovary syndrome., announced the launch of its hormonal diagnostic tool. The test is designed to uncover and address a set of hormonal and metabolic markers, providing clarity around a range of symptoms - from physical symptoms like weight gain and acne to “invisible” symptoms like fatigue and anxiety.
- In November 2020, OmegaQuant, the leader in omega-3 testing, launched a vitamin D test with a sample collection kit that allows one to test for vitamin D deficiency from home. The simple, safe, and convenient test requires just a finger stick and a couple drops of blood for analysis similar to its range of omega-3 blood tests.
Agreements:
- In January 2023, Atomo Diagnostics, an Australia-based diagnostic company, entered into a commercialization agreement with NG Biotech SAS is a developer of rapid point-of-care diagnostics intended to improve decision-making processes in healthcare, to manufacture and distribute rapid blood-based pregnancy tests for both home and professional use in prominent markets. The new pregnancy test combines NG Biotech SAS’s manufactured “highly sensitive hCG test assay” and Atomo’s integrated Pascal blood test device.
- In February 2021, GRAIL, Inc., a healthcare company, announced an agreement with Quest Diagnostics, the leading provider of diagnostic information services, to provide phlebotomy services to support Galleri, GRAIL, Inc’s multi-cancer early detection blood test
Top Companies in the Global Cellular Health Screening Test Market:
- Genova Diagnostics
- Cell Science Systems
- Life Length
- Repeat Diagnostics
- Titianovo
- DNA Labs India
- Segterra
- Telomere Diagnostics
- Spectracell Laboratories
- Zimetry LLC
- Immundiagnostik AG
- Cleveland HeartLab, Inc.
- DNA Diagnostics Center
- Immuno Diagnostics
- Cell Science Systems Corporation
- Life Length S.L.
- Repeat Diagnostics Inc.
- SpectraCell Laboratories
- Telomere Diagnostics
- Bloom Diagnostics
- Kindbody
- GRAIL, Inc
- Atomo Diagnostics
- OmegaQuant
- Allara
- Câ‚‚N Diagnostics
Definition: Cellular health screening tests provide insights into an individual's health at the cellular level by analyzing various biomarkers. There are two main types of cellular health screening tests - clinical testing and research testing. Clinical testing looks at established biomarkers that can indicate preconditions to various diseases. Some examples are telomere length testing, heavy metals screening, nutritional status testing, and inflammation marker analysis. These tests are meant to catch issues early before clinical symptoms appear so that lifestyle or diet changes can be made. The advantage is a more proactive approach to health management, however the disadvantage is that clinical utility is still being established for some tests
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
Select a License Type
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients




