Cardiogenic Shock Treatment Market Size and Trends
The global cardiogenic shock treatment market is estimated to be valued at USD 1.18 Bn in 2025 and is expected to reach USD 1.93 Bn by 2032, exhibiting a compound annual growth rate (CAGR) of 7.3% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
The market is witnessing various innovations that can help in the effective treatment of cardiogenic shock. Companies are investing heavily in the research and development of advanced drugs and devices. Furthermore, growing awareness about the early diagnosis and treatment of cardiogenic shock are expected to create new opportunities in the coming years. Adoption of minimally invasive techniques is also picking up owing to the benefits such as lesser trauma, fewer complications, and shorter hospital stay.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
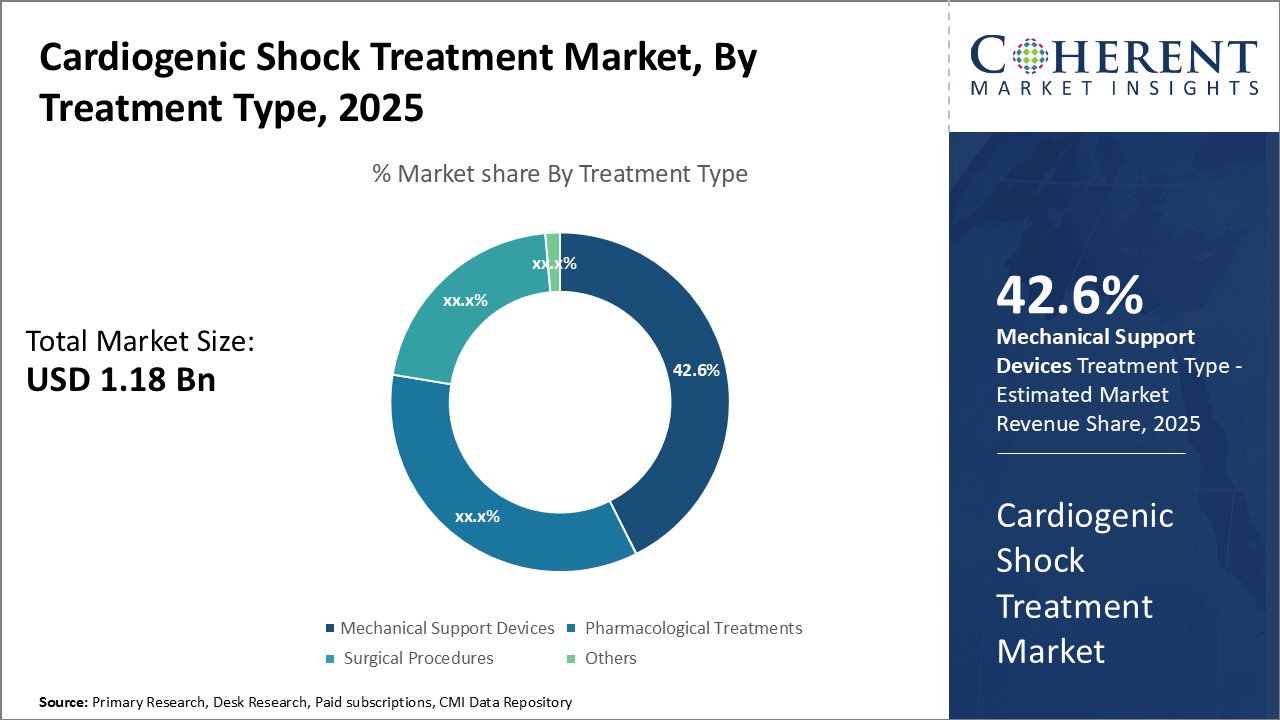
Insights By Treatment Type - Advancements in device technology drive demand for mechanical support devices
In terms of treatment type, mechanical support devices segment is expected to contribute the highest market share of 42.6% in 2025 owing to advancements in device technology. Mechanical support devices are relied upon heavily in the treatment of cardiogenic shock as they can sustain the patient's vital organs until recovery. Key devices like ventricular assist devices (VADs) and intra-aortic balloon pumps (IABPs) have seen continual innovation, improving outcomes. VADs in particular have emerged as a viable long-term solution, helping patients regain mobility and functionality.
Insights By End User - Hospitals lead with 24/7 care and ICU support
Hospitals segment is expected to contribute the highest market share of 40.62% in 2025 owing to their round-the-clock infrastructure and intensive care facilities. Cardiogenic shock necessitates urgent specialized treatment to stabilize critically ill patients. Hospitals are best equipped to provide such dedicated care effectively. Most cardiogenic shock cases result from severe heart attacks, cardiac surgeries or trauma, and demand advanced life support. Only hospitals maintain specialized equipment, experienced intensive care staff, and multidisciplinary cardiology teams pivotal for attending to hemodynamic instability and organ dysfunction promptly.
Regional Insights
Need a Different Region or Segment? Download Free Sample
Regional Analysis: Global Cardiogenic Shock Treatment Market
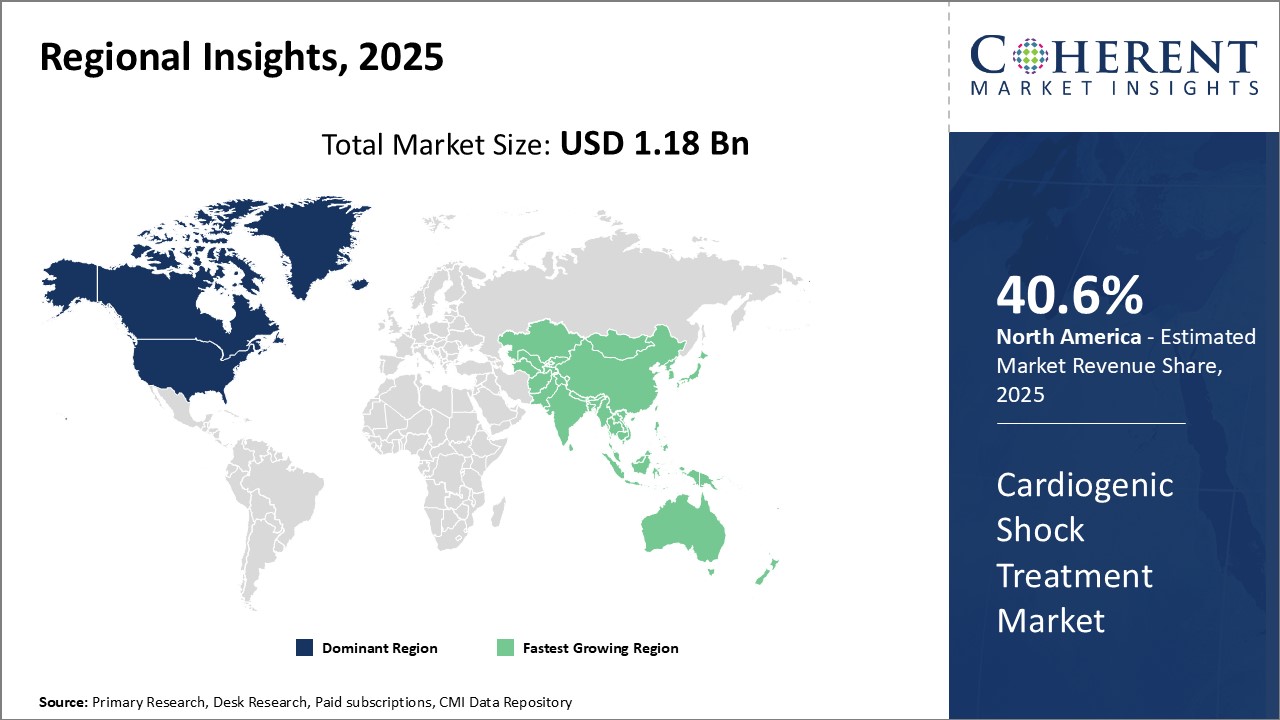
Dominating Region: North America
North America dominates the market with an estimated share of 40.6% in 2025. This can be attributed to factors such as well-established healthcare infrastructure, higher healthcare spending, and the presence of leading market players. The region has a favorable regulatory environment that helps expedite clinical trials.
Fastest-Growing Region: Asia Pacific
The Asia Pacific region exhibits the fastest growth due to the increasing incidences of cardiovascular diseases and growing medical tourism across developing countries. Improving access to healthcare and widespread health insurance coverage in many countries contribute to the rapid expansion of the market.
Cardiogenic Shock Treatment Market Outlook for Key Countries
U.S.: Advancing Cardiogenic Shock with New Launches
The U.S. cardiogenic shock treatment market is fueled by new product approvals and launches. Companies like Abbott Laboratories have strengthened their product portfolios catering to the cardiogenic shock patient population. In October 2022, the American Heart Association (AHA) launched the Cardiogenic Shock Registry under Get With The Guidelines to study symptoms, treatment, and outcomes of shock.
China: Prioritizing Cardiogenic Shock Under Healthy China 2030
China's cardiogenic shock treatment market is projected to rise steadily considering the government's focus on addressing the growing burden of heart disease. For instance, in January 2023, the Chinese government's Healthy China 2030 plan emphasizes the prevention and treatment of cardiovascular diseases (CVDs), which includes addressing cardiogenic shock as a critical area of focus. CVDs account for about 40.5% of all deaths in China, highlighting the urgent need for effective treatments.
Japan: Innovating Cardiovascular Care with Advanced Technologies
Japan leads in cardiovascular drug innovation, with companies like Toray Industries and Terumo Corporation, driving advancements in left ventricular assist devices (LVADs) and technologies essential for managing cardiogenic shock. According to data published by ScienceDirect in September 2025, Japan's healthcare system is renowned for its high standards and seamless integration of advanced medical technologies, enabling effective management of cardiogenic shock.
India: Enhancing Access to Cardiogenic Shock Treatments
India's cardiogenic shock treatment market is witnessing higher investment from global medical technology giants to address the large unmet needs and enhance availability of advanced treatment options in both public and private healthcare settings. For instance, according to Express Healthcare, in September 2024, cardiogenic shock, a severe complication of heart disease, is prevalent in India due to the high burden of coronary artery disease. Advanced treatments like Intra-Aortic Balloon Pump (IABP) and Extracorporeal Membrane Oxygenation (ECMO) are critical but limited to urban centers, while rural areas face challenges in access.
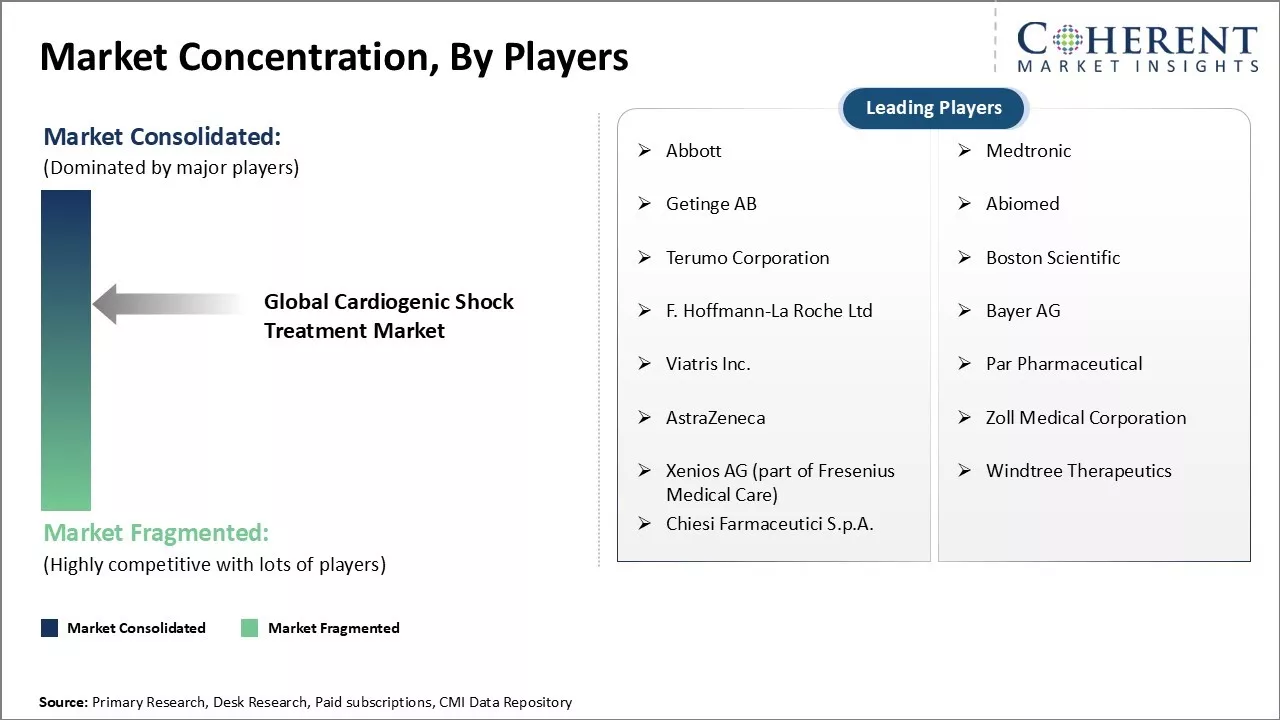
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Top Strategies Followed by Global Cardiogenic Shock Treatment Market Players
- Established Players: Leading companies heavily invest in research and development to drive innovation. For example, major players like Abbott and Medtronic invest over 15% of annual revenue in R&D. This allows them to develop cutting-edge products like left ventricular assist devices (LVADs) that improve clinical outcomes.
- Mid-Level Players: Mid-sized companies strive to deliver quality solutions at competitive prices. They focus on affordable product lines targeting budget-conscious hospitals and patients. For instance, BiVACOR develops cost-effective temporary left ventricular assist device for cardiogenic shock recovery.
- Small-Scale Players: Small players survive by occupying specialized market segments. Some target rare diseases like fulminant myocarditis. Others focus on underserved regions like Africa, India, and Latin America through locally produced technologies. This narrow targeting allows niche survival.
Emerging Startups in the Global Cardiogenic Shock Treatment Market
Innovative Technologies: Startups are developing cutting-edge solutions. Circulation is trailing a smart total artificial heart that self-regulates using AI. Hearthero uses sensors to non-invasively assess cardiac function through a mobile app. These innovative technologies could transform cardiogenic shock management.
Sustainable Solutions: Several startups focus on sustainability. Neousys employs recycled materials in ventricular assist devices to lower costs and waste. CardioRen uses plant-based biomaterials and bioresorbable scaffolds to offer eco-friendly alternatives. Their products may aid sustainability goals in healthcare.
Market Contribution: Startups fulfill unmet needs. Transmedics develops organ care systems to extend allocation for heart transplantation. CoreoGraft provides biodegradable implants for congenital heart defects treatment.
Cardiogenic Shock Treatment Industry News
- On October 24, 2024, Abbott, a pharmaceutical company, announced the TEAM-HF clinical trial, aiming to improve outcomes for patients with worsening heart failure. The study will enroll up to 850 patients across 75 sites and use Abbott's CardioMEMS HF System to measure pulmonary artery pressures, identifying high-risk patients who could benefit from early use of the HeartMate 3 LVAD. The trial seeks to optimize timing for advanced therapies, enhancing survival and care for heart failure patients.
- On August 9, 2024, the U.S. Food and Drug Administration approved neffy (epinephrine nasal spray) as the first non-injectable treatment for life-threatening allergic reactions (anaphylaxis) in adults and children weighing at least 66 pounds. Neffy delivers comparable effectiveness to injectable epinephrine and addresses barriers to rapid treatment, especially for patients who avoid injections.
- On May 20, 2024, Avenacy, a specialty pharmaceutical company, announced the U.S. launch of Isoproterenol Hydrochloride Injection, USP, a generic equivalent of ISUPREL, approved by the U.S. FDA. The injection treats conditions like heart block, cardiac arrest, bronchospasm during anesthesia, and cardiogenic shock. Available in 0.2 mg/mL and 1 mg/5 mL single-dose vials, it features differentiated packaging for accurate medication selection. Shipping begins this week through Avenacy’s FDA-compliant global manufacturing network.
- In December 2022, Abiomed, a medical device company’s Impella RP Flex with SmartAssist received U.S. Food and Drug Administration (FDA) pre-market approval on October 31, 2022, for treating acute right heart failure for up to 14 days. Implanted via the internal jugular vein, it enables mobility and uses dual-sensor technology for optimized management. Clinical studies show early use improves survival rates significantly. The device simplifies anticoagulant management and remains the only U.S. Food and Drug Administration-approved mechanical circulatory support (MCS) for right heart failure, addressing a critical need in underdiagnosed patients.
Key Takeaways from Analyst
- One of the major drivers for this growth is the rising prevalence of cardiovascular diseases worldwide. Cardiogenic shock can occur as a complication of various heart conditions like heart attack, heart failure, etc. which are becoming more common. Another factor is the improving reimbursement scenario for heart failure and heart attack treatments in various developed and developing countries. This will make advanced cardiogenic shock treatments more affordable for patients and increase demand.
- However, the high costs associated with cardiogenic shock medications and devices are expected to restrain the market growth to some extent. Cardiogenic shock requires intensive care and management which involves the use of costly drugs and lifesaving equipment. This acts as a barrier, especially in price-sensitive developing regions. North America is expected to continue dominating the global cardiogenic shock treatment market during the forecast period owing to the advanced healthcare infrastructure and high healthcare expenditure in the region. Within North America, the U.S. accounts for the major share due to presence of prominent players. Asia Pacific is poised to be the fastest-growing market due to rising healthcare investments, growing medical tourism, and increasing burden of heart diseases in countries like China and India.
Market Report Scope
Cardiogenic Shock Treatment Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.18 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.3% | 2032 Value Projection: | USD 1.93 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Abbott, Medtronic, Getinge AB, Abiomed, Terumo Corporation, Boston Scientific, F. Hoffmann-La Roche Ltd, Bayer AG, Viatris Inc., Par Pharmaceutical, AstraZeneca, Zoll Medical Corporation, Xenios AG (part of Fresenius Medical Care), Windtree Therapeutics, and Chiesi Farmaceutici S.p.A. |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Dynamics
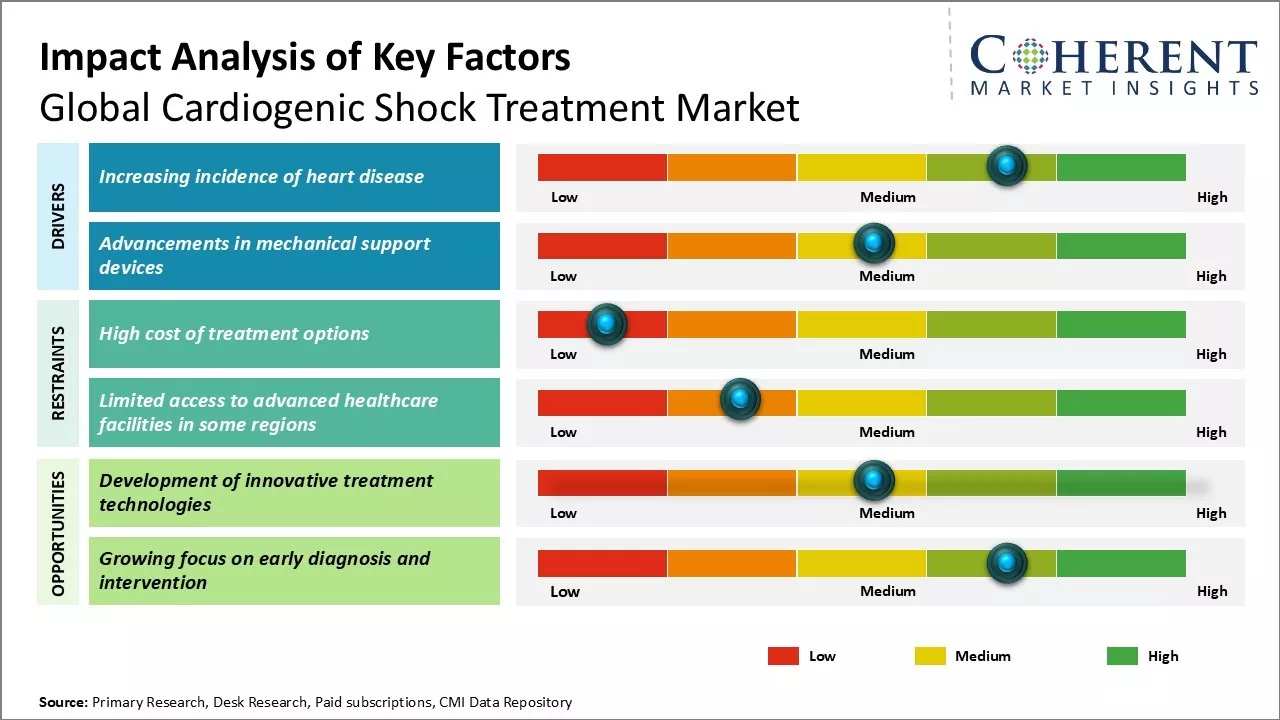
Market Driver - Increasing incidence of heart disease
The rise in cardiac risk factors like diabetes, high blood pressure, and smoking has led to a global surge in heart disease cases. According to the National Library of Medicine in November 2021, cardiogenic shock is one of the most severe complications, affecting 5–10% of heart attack cases and carrying a 50–80% mortality rate if untreated. Aging populations, unhealthy lifestyles, and limited awareness in remote areas further drive the demand for advanced cardiogenic shock treatments, including drugs and mechanical support devices. In May 2023, the World Heart Federation revealed a rise in global cardiovascular disease (CVD) deaths from 12.1 million in 1990 to 20.5 million in 2021, with most deaths occurring in low- and middle-income countries. While CVD death rates declined globally, high-income countries saw faster progress.
Market Challenge - High cost of treatment options
One of the key challenges being faced by the global cardiogenic shock treatment market is the high cost associated with treatment options available. Cardiogenic shock needs immediate medical attention and the therapies and drugs used are generally very expensive. The devices and equipment required for surgeries, heart transplants, and other intensive care also contribute significantly to the overall treatment cost. This makes cardiogenic shock treatment unaffordable for many people, especially in developing regions where access to healthcare is limited.
Market Opportunity - Development of innovative treatment technologies
The global cardiogenic shock treatment market has considerable potential for growth with the development of innovative treatment technologies. There is a rising need for new therapies that can provide more targeted intervention and better clinical outcomes at lower costs. This demand presents a major opportunity for market players to invest in R&D and develop cutting-edge technologies. Areas such as advanced surgical robotics, regenerative medicines, non-invasive procedures, and patient-specific treatment planning offer promising scopes of innovation.
Key Stakeholders of Market
What does Growth in the Cardiogenic Shock Treatment Market mean for Different Stakeholders?
The cardiogenic shock treatment market has multiple players with varied designations and offers multiple opportunities based on their scope of operations.
|
Key Pharmaceutical Stakeholder |
Opportunities Due to Cardiogenic Shock Treatment Growth |
|
Retail Pharmacies |
Expansion of product offerings to include new drugs and personalized medicine solutions, enhancing customer care and market reach. |
|
Chemical Suppliers |
Growth in demand for specialty chemicals used in drug synthesis, including organic intermediates, catalysts, and reagents. |
|
Pharmaceutical Companies |
Expansion of product pipelines with new drug discoveries, biologics, and biosimilars, capitalizing on growing global healthcare needs. |
|
Contract Research Organizations (CROs) |
Increased outsourcing of clinical trials and drug development, offering opportunities for growth and long-term partnerships. |
|
Contract Manufacturing Organizations (CMOs) |
Growing demand for scalable manufacturing solutions, including biologics production and complex drug formulations. |
|
Raw Materials Suppliers |
Increased demand for high-quality active pharmaceutical ingredients (APIs) and excipients to support drug formulation and production. |
|
Healthcare Providers |
New treatment options and innovative therapies, improving patient care and expanding healthcare services. |
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Market Segmentation
- Treatment Type Insights (Revenue, USD Bn, 2020 - 2032)
-
- Mechanical Support Devices
- Pharmacological Treatments:
- Surgical Procedures
- Others
- End User Insights (Revenue, USD Bn, 2020 - 2032)
-
- Hospitals
- Cardiac Care Centers
- Ambulatory Surgical Centers
- Others
- Regional Insights (Revenue, USD Bn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Abbott
- Medtronic
- Getinge AB
- Abiomed
- Terumo Corporation
- Boston Scientific
- F.Hoffmann-La Roche Ltd
- Bayer AG
- Viatris Inc.
- Par Pharmaceutical
- AstraZeneca
- Zoll Medical Corporation
- Xenios AG (part of Fresenius Medical Care)
- Windtree Therapeutics
- Chiesi Farmaceutici S.p.A.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
