Cardiac Arrhythmia Monitoring Devices Market Analysis & Forecast: 2025-2032
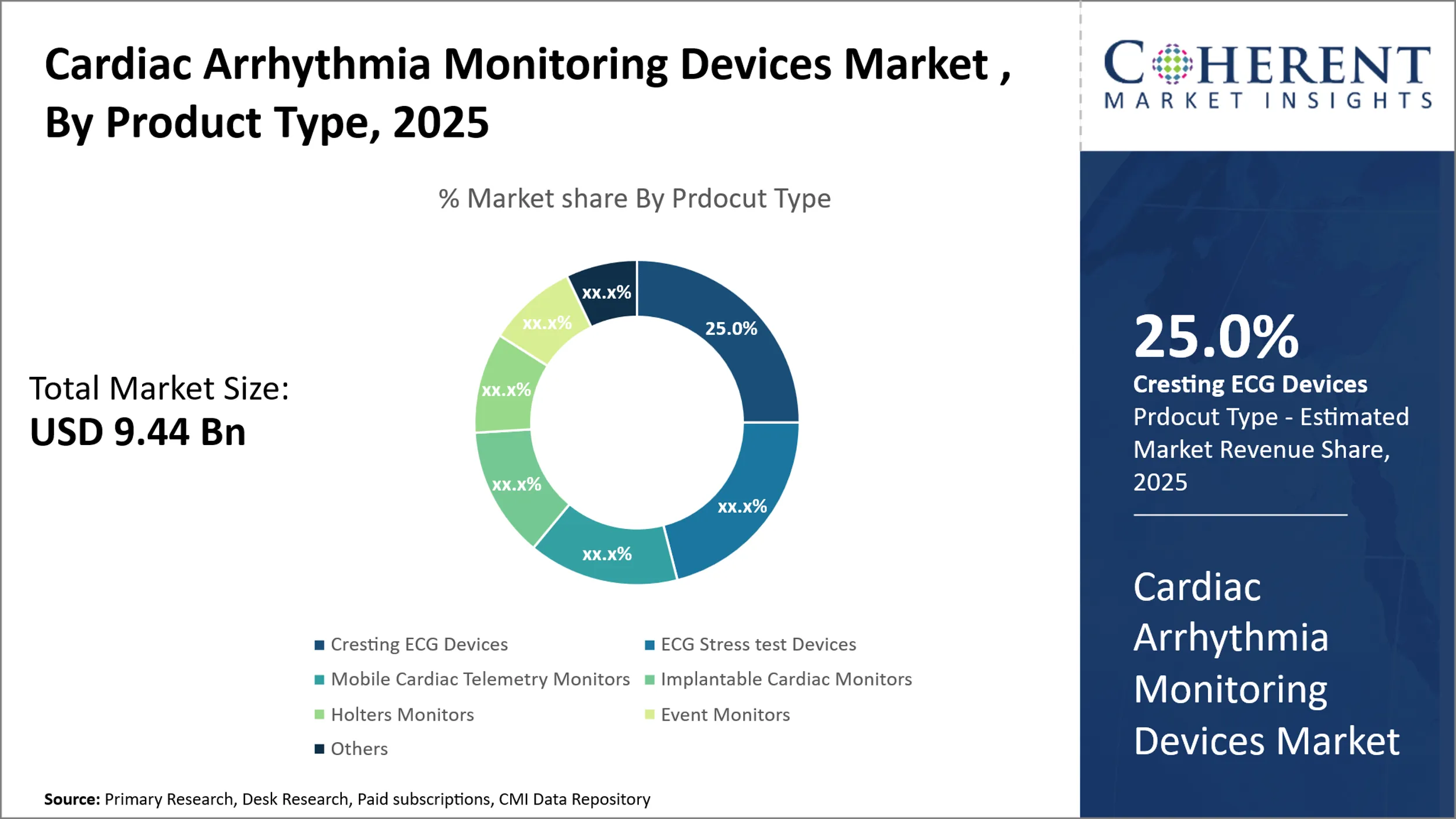
Cardiac Arrhythmia Monitoring Devices Market is estimated to be valued at USD 9.44 Bn in 2025 and is expected to reach USD 15.97 Bn in 2032, exhibiting a compound annual growth rate (CAGR) of 7.8% from 2025 to 2032.
Key Takeaways
- According to Product Type, the Resting ECG Device category is anticipated to account for the largest share of the Cardiac Arrhythmia Monitoring Devices market in 2025.
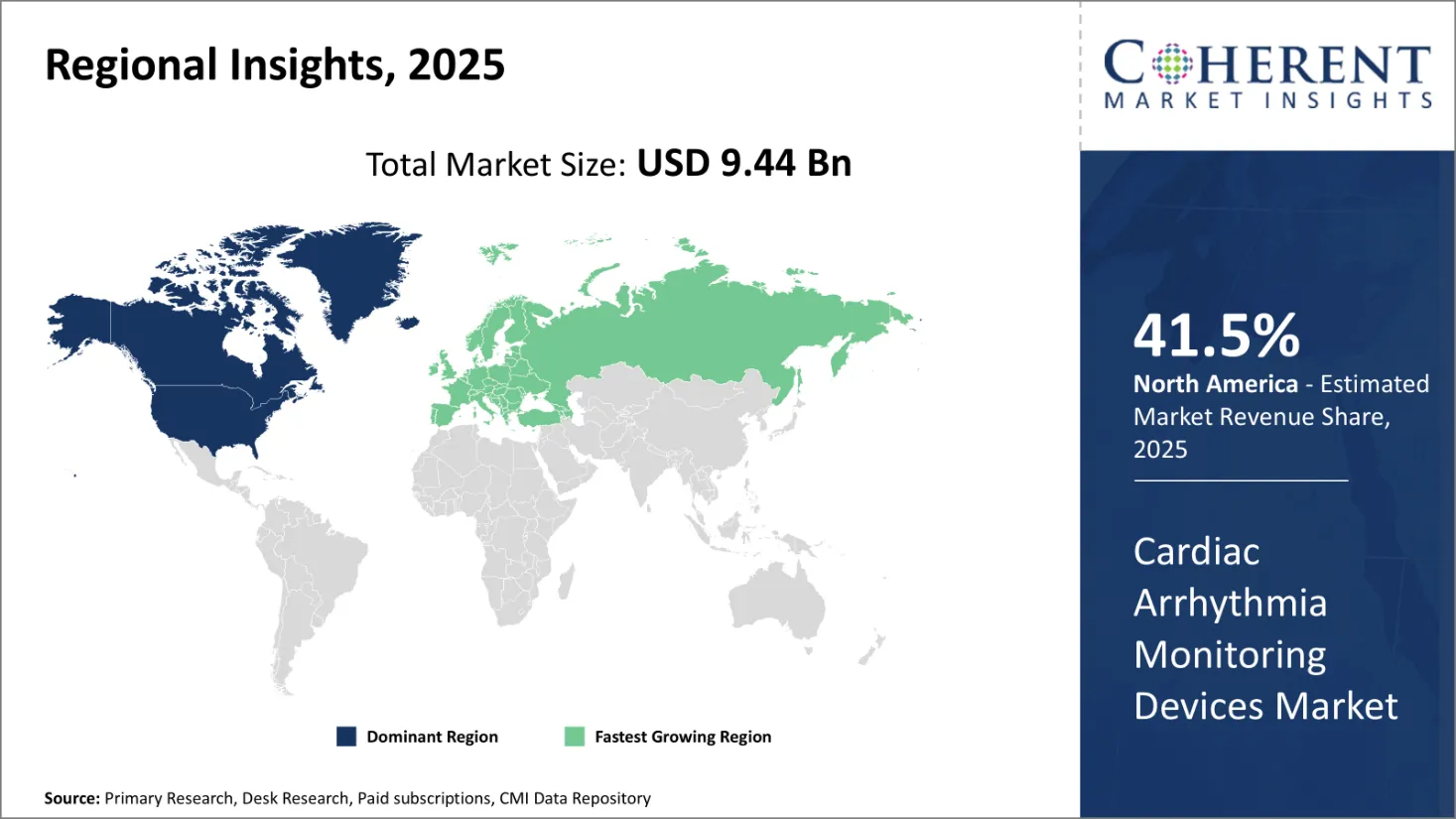
- According to Region, North America also holds the largest market share of 41.5% for Cardiac Arrhythmia Monitoring Devices market growth by 2025.
- Europe is considered to be the second dominating region with 29.7% market share for Cardiac Arrhythmia Monitoring Devices market by 2025.
- Asia Pacific is considered to be the third largest region with 10.2% market share by 2025 for Cardiac Arrhythmia Monitoring Devices market.
Market Overview
The global Cardiac Arrhythmia Monitoring Devices Market Size is growing due to increasing usage of cardiac arrhythmia monitoring devices to detect irregular heart rhythms and monitor heart activity. These devices include resting ECG devices, ECG stress test devices, mobile cardiac telemetry monitors, plantable cardiac monitors, holter monitors, and event monitors among others.
For instance, in May 2025, iRhythm Technologies, Inc. announced the commercial launch in Japan of its Zio long-term continuous ECG monitoring (LTCM) system, commercially introduced in this market as the Zio ECG Recording and Analysis System.
Current Events and Its Impact on the Cardiac Arrhythmia Monitoring Devices Market
|
Event |
Description and Impact |
|
Growing Use of Wearable and Remote Monitoring Technology |
|
|
Expansion of Telehealth Services |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Role of AI (Artificial Intelligence) in Cardiac Arrhythmia Monitoring Devices Market
Artificial intelligence (AI) plays a critical role in advancing cardiac arrhythmia monitoring by enabling faster, more accurate detection and diagnosis. AI algorithms analyze large volumes of ECG data to identify abnormal heart rhythms with high precision, reducing the risk of missed or false diagnoses. These systems can automatically flag potential arrhythmias, allowing clinicians to focus on complex cases and improve patient outcomes. In long-term monitoring devices, AI enhances efficiency by filtering noise, prioritizing clinically relevant events, and generating detailed reports.
In May 2025, iRhythm Technologies launched its Zio® ECG Recording and Analysis System in Japan. This long-term continuous ECG monitoring system offers up to 14 days of uninterrupted monitoring and uses a PMDA-approved AI algorithm for advanced arrhythmia detection. It marks a major improvement over traditional 24–48 hour Holter monitors and other patch-based systems limited to 7 days.
Cardiac Arrhythmia Monitoring Devices Market Insights, by Product type
In terms of product type, the resting ECG devices segment accounted for the largest share of the market in 2025. Resting ECG devices are portable and easy-to-use for diagnosis of heart conditions, making them a commonly adopted product.
For instance, in November 2024, SCHILLER launched the new medilogFD Holter ECG device. Superior resting ECG-grade 128,000Hz sampling rate on 12 leads for real-time P-wave analysis, sophisticated artifact suppression, and motion detection, enabling a speedy evaluation.
Cardiac Arrhythmia Monitoring Devices Market Insights, by Application
In terms of type, the Atrial Fibrillation segment is expected to contribute largest share of the Cardiac Arrhythmia Monitoring Devices market trends in 2025. Early and accurate AF detection reduces the risk of stroke and other complications, leading to better patient outcomes and lower healthcare costs, further cementing the AF segment’s priority in the target market.
For instance, in May 2025, Abbott, the global healthcare company, announced the launch of the TactiFlex Sensor Enabled Ablation Catheter, the world’s first ablation catheter with a flexible tip and contact force technology.
Regional Insights
To learn more about this report, Download Free Sample
North America Cardiac Arrhythmia Monitoring Devices Market
North America is expected to be the largest market for cardiac arrhythmia monitoring devices market during the forecast period, accounting for over 41.5% of the market share in 2025. The growth of the market in North America is attributed to the high incidence of cardiac diseases, established healthcare infrastructure, and presence of key players in the region.
For instance, in May 2025, HeartBeam, Inc., a medical technology company focused on transforming cardiac care by providing powerful cardiac insights, today announced that the US Food and Drug Administration (FDA) has granted 510(k) clearance of the HeartBeam system for comprehensive arrhythmia assessment.
Asia Pacific Cardiac Arrhythmia Monitoring Devices Market
The Asia Pacific market is expected to be the fastest-growing market for cardiac arrhythmia monitoring devices market, with a CAGR of over 10.2% during the forecast period. The growth of the market in Asia Pacific is attributed to the large patient pool, improving healthcare infrastructure, high unmet needs, and growing awareness regarding advanced cardiac monitoring devices in the region.
For instance, in June 2023, BIOTRONIK, a leader in implantable medical device technology, today announced the first global implantation of its BIOMONITOR IV implantable cardiac monitor (ICM). The procedure was conducted by Luigi Di Biase, MD, PhD, Section Head of Electrophysiology, Director of Arrhythmia Services and Professor of Medicine at Montefiore Einstein in the Bronx, NY. This first implant marks the next step in the advancement of cardiac monitoring technology.
Cardiac Arrhythmia Monitoring Devices Market Dominating Countries
U.S Cardiac Arrhythmia Monitoring Devices Market
The rising incidence of heart-related disorders, a robust healthcare system, and the quick uptake of cutting-edge diagnostic technologies are driving the U.S. market. Almost 697,000 deaths are attributed to cardiovascular illness each year, which highlights the necessity for efficient arrhythmia monitoring systems.
India Cardiac Arrhythmia Monitoring Devices Market
The Indian market is expanding rapidly due to a rising burden of cardiovascular diseases, especially arrhythmias, and a growing elderly population. Urbanization and the expansion of private cardiology networks are increasing access to advanced diagnostics.
Market Report Scope
Cardiac Arrhythmia Monitoring Devices Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 9.44 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 7.8% | 2032 Value Projection: | USD 15.97 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Medtronic, Abbott Laboratories, Boston Scientific, Biotronik, Koninklijke Philips, Nihon Kohden, Fukuda Denshi, Hill-Rom Holdings, Mindray Medical, Schiller AG, Spacelabs Healthcare, GE Healthcare, Cardiac Science Corporation, Lifewatch AG, Beijing Choice Electronic Tech Co, Vivaquant, Preventice Solutions, iRhythm Technologies, Applied Cardiac Systems and BioTelemetry |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Cardiac Arrhythmia Monitoring Devices Market: Growth Drivers
- Growing prevalence of cardiovascular diseases: The rising prevalence of cardiovascular diseases such as arrhythmias, strokes, and heart failure is a major factor driving the growth of the cardiac arrhythmia monitoring devices market. The incidence of atrial fibrillation, a common cardiac arrhythmia, is increasing globally.
- Favorable reimbursement policies: Favorable reimbursement policies offered by private insurance companies as well as government health agencies are propelling the adoption of cardiac arrhythmia monitoring devices. For instance, in the U.S., Medicare provides coverage for Holter monitoring tests under Part B.
- Technological advancements in cardiac monitoring: Advancements in wearable technology, remote monitoring, artificial intelligence (AI), and big data analytics are leading to the launch of innovative cardiac arrhythmia monitoring products. Key players are integrating advanced features like extended wearability, data connectivity, predictive algorithms, and EMR integration in their new product offerings.
- Growing focus on preventive care: The growing focus on preventive care and early diagnosis is boosting the use of cardiac monitors for proactive heart health management. Ambulatory cardiac telemetry devices are gaining popularity for long-term heart rhythm monitoring in high-risk patients, to detect asymptomatic arrhythmic events and prevent complications like stroke.
Cardiac Arrhythmia Monitoring Devices Market: Trends
- Shift towards remote patient monitoring: A major trend in the market is the increasing utilization of remote cardiac monitoring services for better arrhythmia management and care coordination. Advanced devices integrated with wireless connectivity allow data transmission to mobile apps and online portals accessible to physicians. This enables timely interventions based on remote ECG analysis. Players are forming partnerships to offer comprehensive RPM services.
- Growing adoption of mobile cardiac telemetry: The adoption of mobile cardiac telemetry (MCT) monitors is rising owing to their ability to provide real-time monitoring for fast arrhythmia detection. MCT devices comprise a small sensor attached to electrodes that wirelessly transmits ECG data to a portable recorder. This enhances monitoring for temporarily high-risk ambulatory patients. The launch of new advanced MCT monitors is supporting this growing trend.
- Technological innovations in smart wearable: Key players are innovating smart wearable products such as ECG-enabled patches, shirts, watches that use advanced sensors and AI to continuously monitor heart activity. For instance, the new Carnation Ambulatory Monitor by Vivalnk, provider of remote patient monitoring services through innovative technology features a machine-learning algorithm to detect arrhythmias from ECG data.
- Shift towards preventive and personalized care: There is a rising trend of using cardiac arrhythmia monitoring devices, such as injectable cardiac monitors (ICMs) and ambulatory ECG, for preventive care in high-risk groups. The use of advanced ECG analytics and digital platforms is also rising to enable monitoring of asymptomatic events and provide individualized care based on patients’ specific needs. This shift towards preventive and personalized care models is shaping market trends.
Cardiac Arrhythmia Monitoring Devices Market: Opportunities
- Emerging markets: The emerging markets in Asia Pacific, Latin America, and Middle East & Africa offer significant untapped opportunities for market players. These regions have a high unmet need for advanced cardiac monitoring devices owing to the rising incidence of arrhythmias, improving healthcare infrastructure, growing middle-class population, and increasing awareness. Key players can focus on expanding their footprint in emerging economies through partnerships, collaborations, and launches of cost-effective products to increase their customer base.
- Integration with remote patient monitoring: The integration of remote patient monitoring functionalities in cardiac arrhythmia devices provides new opportunities for growth. Key players are developing next-gen products with cellular or wireless connectivity and compatible mobile apps to enable seamless data transmission and continuous patient monitoring from home settings. This can help improve diagnosis and management for patients in remote locations.
- Big data analytics: The application of big data analytics and AI represents an opportunity for companies to develop smart cardiac monitors with predictive capabilities. By analyzing data from ECG recordings combined with patient health records, devices can be enabled to provide actionable insights, predict arrhythmia episodes using algorithms, and recommend interventions. This can aid in preventive care and personalized treatment.
- Growing use of wearables: With the rising adoption of smartwatches and fitness bands with heart rate monitoring features, there is an opportunity for companies to integrate medical-grade heart rhythm tracking into consumer wearables. They can partner with wearable firms to develop ECG-enabled smartwatches and bands to monitor arrhythmias in ambulatory settings for extended periods. This can help with mass screening and diagnosis.
Analyst Opinion
- Over the next five years, there is significant growth potential in the market for cardiac arrhythmia monitoring devices. The two main factors driving the need for arrhythmia monitoring devices worldwide are the rising incidence of cardiovascular illnesses and the aging of the population. However, the high expense of intricate cardiac arrhythmia monitoring procedures may somewhat limit market expansion.
- The market is now dominated by North America, especially the United States, because of significant investments in innovation and a large patient population. Nonetheless, during the course of the projected period, the Asia Pacific region is anticipated to increase at the fastest rate. This is explained by expanding healthcare spending, the expansion of the medical tourism sector, and the growing attention of major firms to take advantage of opportunities in China's and India's developing economies.
- Technologies for remote and wearable monitoring create new avenues for treating cardiac arrhythmias. Device manufacturers have been able to create cutting-edge wireless monitoring systems thanks to the widespread use of smartphones and smart watches. This has improved accessibility to cardiac arrhythmia monitoring and increased convenience. Additionally, as medical technology advances, the diagnosis and treatment of cardiac arrhythmias become more efficient and less expensive over time.
- In summary, the market for cardiac arrhythmia monitoring devices has solid foundations and a number of tailwinds that will support sustained expansion on a global scale over time. Demand will be further stimulated by ongoing technical advancements and market growth initiatives by industry participants in developing regions.
Cardiac Arrhythmia Monitoring Devices Market: Key Developments
- May 2025, Johnson & Johnson MedTech, a global leader in cardiac arrhythmia treatment, announced the U.S. launch of the SOUNDSTAR CRYSTAL™ Ultrasound Catheter for intracardiac echocardiography (ICE) imaging in cardiac ablation procedures.
- July 2024, FibriCheck, a Belgium-based healthcare technology company, gained U.S. Food and Drug Administration (FDA) for its artificial intelligence-powered digital platform that uses smartphone cameras to obtain heart rhythm measurements. This FDA clearance covers FibriCheck’s entire platform, including its AI algorithms, smartphone application and provider portal.
- May 2024, Vivalink, a leading provider of digital healthcare solutions, unveiled its comprehensive technology solution designed for Mobile Cardiac Telemetry (MCT) and Holter monitoring. The solution integrates remote patient monitoring (RPM) technologies and advanced arrhythmia detection algorithms, streamlining deployment and enhancing patient care to meet the increasing demand for efficient ambulatory ECG monitoring solutions.
Cardiac Arrhythmia Monitoring Devices Market: Key Companies
Some of the key players in the global Cardiac Arrhythmia Monitoring Devices market are Medtronic, Abbott Laboratories, Boston Scientific, Biotronik, Koninklijke Philips, Nihon Kohden, Fukuda Denshi, Hill-Rom Holdings, Mindray Medical, Schiller AG, Spacelabs Healthcare, GE Healthcare, Cardiac Science Corporation, Lifewatch AG, Beijing Choice Electronic Tech Co, Vivaquant, Preventice Solutions, iRhythm Technologies, Applied Cardiac Systems and BioTelemetry.
Market Segmentation
- By Product Type
- Resting ECG Devices
- ECG Stress Test Devices
- Mobile Cardiac Telemetry Monitors
- Implantable Cardiac Monitors
- Holter Monitors
- Event Monitors
- Others (Wearable ECG Monitors, Smart ECG Monitors)
- By Application
- Bradycardia
- Tachycardia
- Atrial Fibrillation
- Premature Contraction
- Conduction Disorders
- Others (Ventricular Fibrillation, Pacing Monitoring)
- By End User
- Hospitals
- Diagnostic Centers
- Ambulatory Surgical Centers
- Homecare Settings
- Others (Research Institutes, Specialty Clinics)
- By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- Key Players
- Medtronic
- Abbott Laboratories
- Boston Scientific
- Biotronik
- Koninklijke Philips
- Nihon Kohden
- Fukuda Denshi
- Hill-Rom Holdings
- Mindray Medical
- Schiller AG
- Spacelabs Healthcare
- GE Healthcare
- Cardiac Science Corporation
- Lifewatch AG
- Beijing Choice Electronic Tech Co
- Vivaquant
- Preventice Solutions
- iRhythm Technologies
- Applied Cardiac Systems
- BioTelemetry
Sources
Primary Research interviews
- Cardiologists and electrophysiologists at leading hospitals
- Biomedical engineers at device companies
- Product managers from digital health startups focused on cardiac monitoring
- Hospital procurement heads or purchasing officers
- Clinical trial investigators in cardiovascular devices
Databases
- U.S. FDA MAUDE & 510(k) Database (for device approvals and adverse events)
- ClinicalTrials.gov (for ongoing and past studies involving arrhythmia monitors)
- WHO Global Health Observatory (for cardiovascular disease statistics)
- OECD Health Statistics
Magazines
- MedTech Dive
- MobiHealthNews
- Medical Design & Outsourcing
- Fierce Biotech
- HealthTech Magazine
- Cardiovascular Business
- Healthcare IT News
Journals
- Journal of the American College of Cardiology (JACC)
- Heart Rhythm Journal
- Circulation: Arrhythmia and Electrophysiology
- Journal of Electrocardiology
- The Lancet Digital Health
- IEEE Transactions on Biomedical Engineering
- European Heart Journal
Newspapers
- The Wall Street Journal – Health & Science Section
- Financial Times – MedTech and Life Sciences
- The Economic Times – Health & Diagnostics (India)
- Nikkei Asia – Medical Devices in APAC
- The New York Times – Health Technology
- Reuters Health
Associations
- American Heart Association (AHA)
- Heart Rhythm Society (HRS)
- European Society of Cardiology (ESC)
- Asia Pacific Heart Rhythm Society (APHRS)
- World Health Organization (WHO) – NCD Division
- Medical Device Innovation Consortium (MDIC)
- AdvaMed – Advanced Medical Technology Association
- Indian Heart Rhythm Society
Proprietary Elements
CMI Data Analytics Tool, Proprietary CMI Existing Repository of information for last 8 years
*Definition: The global cardiac arrhythmia monitoring devices market refers to the industry and domain associated with medical devices used to detect abnormal heart rhythms and monitor cardiac activity. These devices play a vital role in the diagnosis and management of cardiac arrhythmias. They include electrocardiogram (ECG) devices, ambulatory cardiac monitoring devices, implantable loop recorders, and other advanced cardiac monitors.
Share
Share
About Author
Komal Dighe is a Management Consultant with over 8 years of experience in market research and consulting. She excels in managing and delivering high-quality insights and solutions in Health-tech Consulting reports. Her expertise encompasses conducting both primary and secondary research, effectively addressing client requirements, and excelling in market estimation and forecast. Her comprehensive approach ensures that clients receive thorough and accurate analyses, enabling them to make informed decisions and capitalize on market opportunities.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients