Bortezomib Market Size and Trends
Global bortezomib market is estimated to be valued at USD 25.7 Mn in 2025 and is expected to reach USD 35.2 Mn by 2032, exhibiting a compound annual growth rate (CAGR) of 4.6% from 2025 to 2032.
Discover market dynamics shaping the industry: Download Free Sample
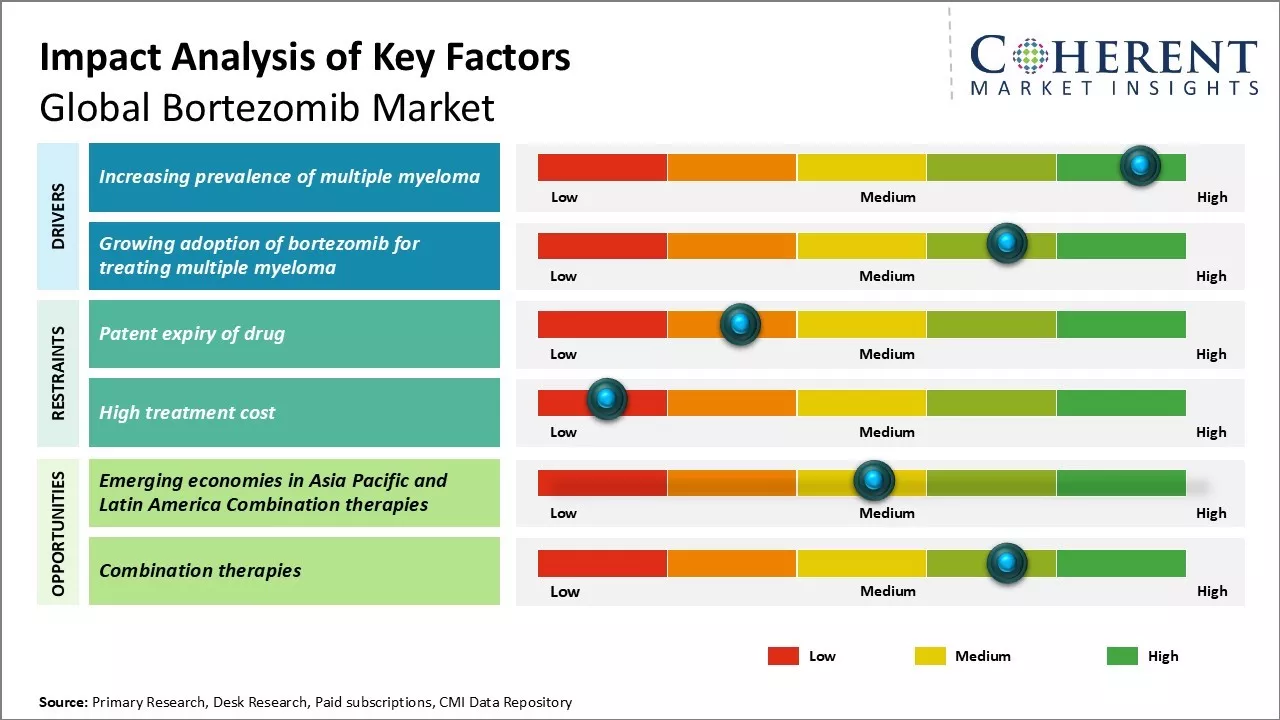
Global bortezomib market growth is driven by rising prevalence of multiple myeloma and growing adoption of bortezomib as a part of combination therapy. Moreover, robust pipeline of novel drug candidates and increasing demand for more effective targeted therapies can offer growth opportunities to market players. However, patent expirations of branded products and development of cheaper generics can hamper the market growth. Continuous research & development to develop safer and more effective drugs through collaborations can drive the market growth.
Increasing prevalence of multiple myeloma
Rising prevalence of multiple myeloma can drive the market growth. Multiple myeloma is a type of cancer that affects plasma cells, which are a type of white blood cell that helps the body to fight infections. The exact cause of multiple myeloma is not known but risk factors include genetics, family history and age. The chances of developing multiple myeloma increases with age and it is very rare in people under age 40 years. As life expectancy rises worldwide, the population of elderly people increases rapidly. Since multiple myeloma is predominantly a disease of older adults, the aging global population directly translates to a larger pool of susceptible individuals.
Rising prevalence of disease boosts demand for drugs for treat multiple myeloma. Currently bortezomib remains one of the most effective and commonly prescribed drugs for multiple myeloma. Multiple myeloma causes bone lesions and destroys bone tissues. It also suppresses the bone marrow and reduces the production of healthy blood cells. If left untreated, multiple myeloma can be fatal within a few months. With rising disease burden, there has been increase in requirement for effective treatment options like bortezomib. Countries with aging populations and better access to healthcare are witnessing a sharp rise in multiple myeloma cases. This growth in patient base has steadily boosted the sales and demand for bortezomib in these regions over the past decade.
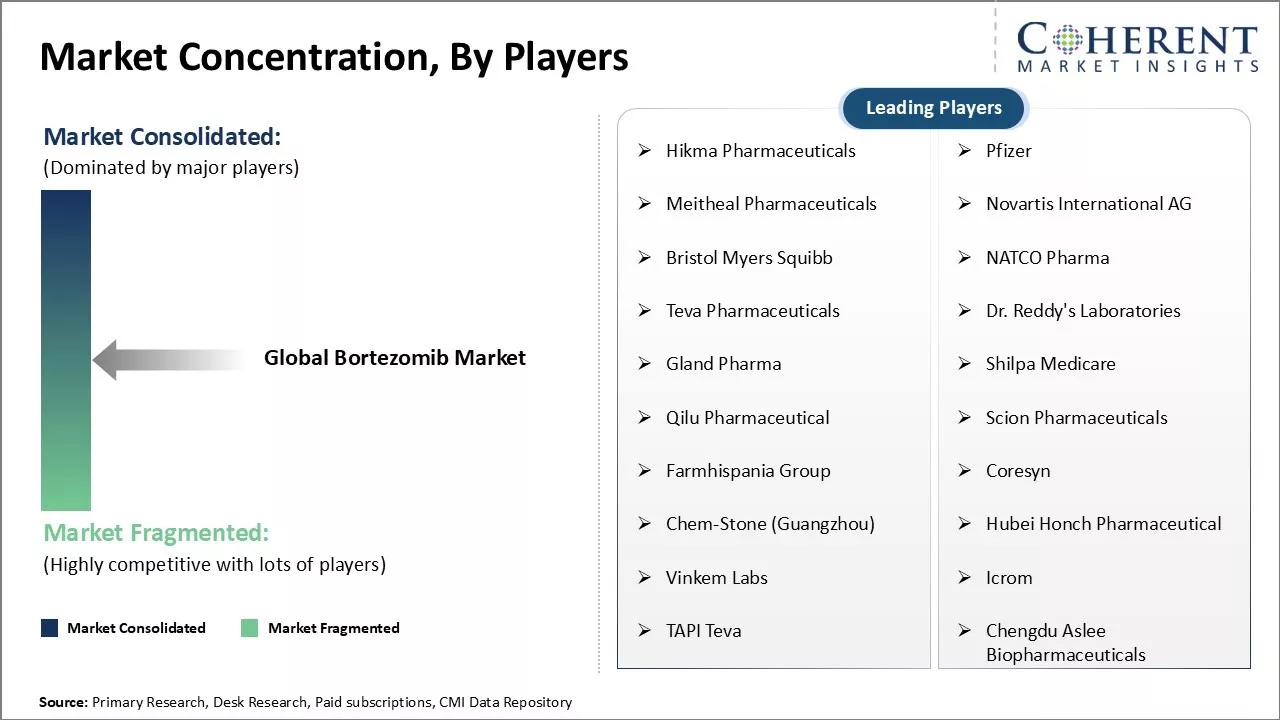
Market Concentration and Competitive Landscape
Get actionable strategies to beat competition: Download Free Sample
Growing adoption of bortezomib for treating multiple myeloma
Bortezomib was the first proteasome inhibitor approved for the treatment of multiple myeloma and it marked a major breakthrough in the management of the disease. Bortezomib is a vital part of the standard treatment regimens for multiple myeloma across all stages and forms. It has demonstrated superiority over other options in improving survival rates and delaying disease progression. Due to its well-established safety profile and significant clinical benefits, bortezomib has gained widespread acceptance amongst both patients and treating physicians over the years. Moreover, availability of bortezomib as both injectable and oral formulations has further increased patient convenience and compliance. Bortezomib is now used at various stages of multiple myeloma including induction therapy, autologous stem cell transplantation, consolidation therapy and for relapsed or refractory multiple myeloma. It is often administered along with other classes of drugs like immunosuppressants to achieve better disease control and response rates. Clinicians continuously expand the scope of bortezomib therapy based on emerging clinical evidence. For example, its use as a maintenance therapy and in combination with immunomodulators are newer approved indications. The improving accessibility to sophisticated treatments and rising affordability have also played a role in augmenting the uptake of bortezomib globally. As more real-world data underpins its clinical utility, adoption is expected to scale to a wider patient pool worldwide in the future.
Key Takeaways from Analyst:
Global bortezomib market growth is driven by increasing prevalence of multiple myeloma globally. As multiple myeloma accounts for about 10% of all hematological cancers, rising healthcare expenditure in developing nations will enable more patients to access bortezomib treatment.
North America currently dominates the market, owing to high adoption of bortezomib in the U.S. for treating multiple myeloma. However, Asia Pacific is expected to emerge as the fastest growing region during the forecast period, due to rapidly developing economies in China and India, where there has been huge number of multiple myeloma cases .
Stringent regulatory policies and the availability of generic versions once patents expire can hamper the market growth. High drug costs also remain a concern for patients in low-income countries. Furthermore, development of new therapies for multiple myeloma will increase competition for bortezomib.
Expanding bortezomib's clinical applications beyond multiple myeloma can offer growth opportunities. Ongoing research on exploring its efficacy for other cancers such as non-Hodgkin's lymphoma and breast cancer can offer growth opportunities.
Market Challenges: Patent expiry of drug
Global bortezomib market growth can be hampered due to patent expiry of the drug. Bortezomib was first approved by the USFDA in 2003 for the treatment of multiple myeloma under the brand name Velcade. However, the patent for Velcade expired in 2020. This paved the way for the entry of several generic versions of bortezomib in the market. The availability of cheaper generic alternatives can bring down the prices of bortezomib therapies. This price decline may reduce the revenues of leading drug manufacturers who have been selling Velcade for years. The expiry of Velcade's patent protection poses a serious revenue and market share threat to these established market players. However, companies are actively working on expanding the approved indications of bortezomib and launching new drug delivery formats to differentiate their products from generics.
Market Opportunities: Emerging Economies in Asia Pacific and Latin America
Emerging economies in Asia Pacific and Latin America can offer market growth opportunities. There has been rise in incidences of blood cancer indications like multiple myeloma in developing nations due to aging population and changing lifestyle trends. In these countries, treatment rates for cancers have been traditionally low due to poor socioeconomic conditions and lack of access to advanced therapies. However, with rising healthcare budgets, greater medical awareness and increasing health insurance penetration, more patients are now gaining access to novel drugs like bortezomib. This presents substantial commercialization possibilities for leading market players. These markets offer the advantage of less stringent regulations and price controls compared to developed markets of North America and Europe. Market players can leverage this environment to establish manufacturing and distribution partnerships with local players in high growth emerging economies.
Discover high revenue pocket segments and roadmap to it: Download Free Sample
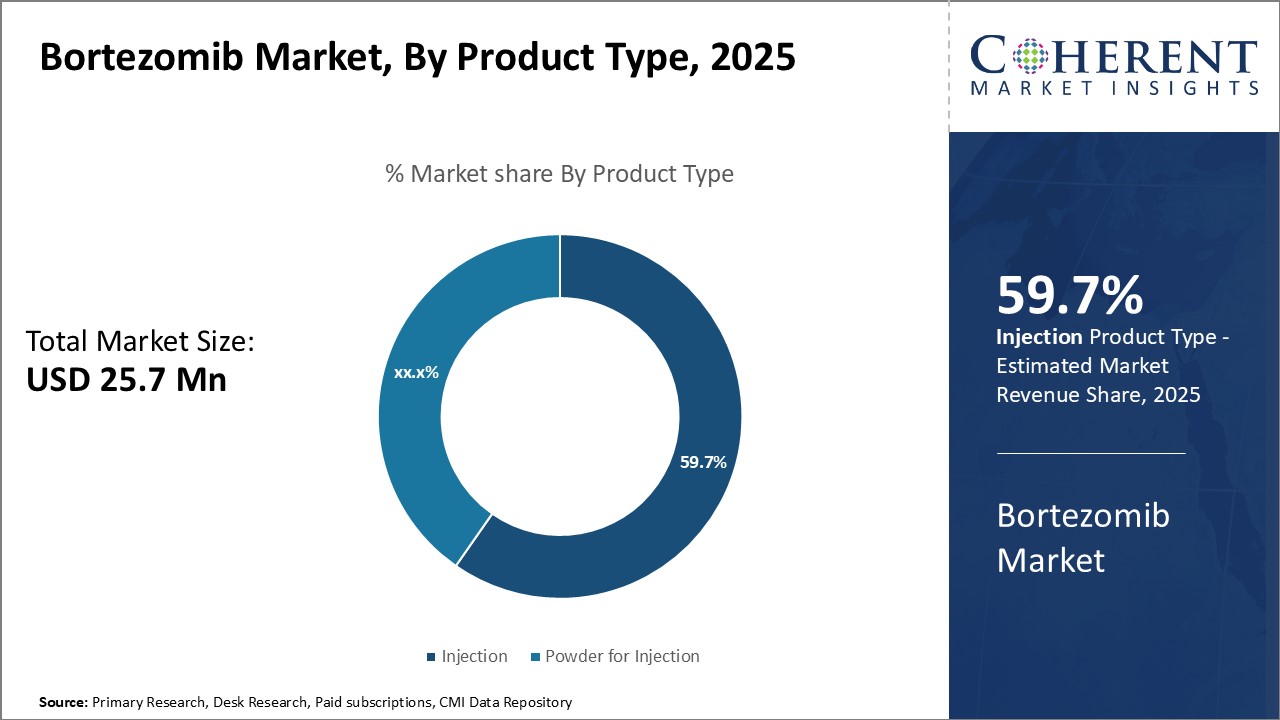
By Product Type- Ever-increasing prescribing rates of Bortezomib injection drives segment growth
In terms of product type, injection segment is estimated to contribute the highest market share of, 59.7% in 2025 owing to its widespread usage across indications. Bortezomib injection is administered through intravenous or subcutaneous routes, allowing for reliable dosing and rapid onset of therapeutic effects. Its clinical efficacy in treating multiple myeloma and other hematologic cancers has been well-established through numerous Phase III trials and real-world usage over the past decade.
Bortezomib injection witness huge adoption by medical oncologists for frontline as well as relapsed/refractory disease settings. In multiple myeloma alone, its use has expanded from second line to now being a standard component of several induction and consolidation regimens. The convenience of injection administration along with its synergistic effects when combined with other anti-myeloma drugs have made it one of the most prescribed therapeutic options worldwide.
Manufacturers are also able to easily offer fixed-dose formulations of Bortezomib injection tailored for different treatment cycles, further, boosting its demand. Wide-scale education initiatives highlighting the drug's clinical utility have additionally raised awareness within the medical community. These combined factors have resulted in sustained high prescription volumes for Bortezomib injection year-over-year.
By Application- Multiple myeloma segment dominates
In terms of application, multiple myeloma segment is estimated to contribute the highest market share of, 48.5% in 2025 owing to its status as the second most common hematologic malignancy after non-Hodgkin's lymphoma. Multiple myeloma arises from plasma cells within the bone marrow and causes problems including anemia, kidney dysfunction and bone fractures or breaks. It represents about 1% of all cancers and over 30,000 new cases are diagnosed annually in the U.S.
Bortezomib has revolutionized multiple myeloma treatment since its approval in the early 2000s. It offers a unique mechanism of action targeting the proteasome pathway, which formed the basis of entirely new clinical regimens. Clinical trials have consistently proven its ability to significantly increase response rates, progression-free survival and overall survival compared to previous standard therapies for multiple myeloma. Due to these widely published benefits, it has become incorporated as the foundation for both frontline and relapsed treatment paradigms in major clinical guidelines.
Growing elderly population globally also contributes to rising multiple myeloma prevalence Prognosis has also improved due to newer treatments like Bortezomib. These factors ensure a steady, high volume of patients receiving Bortezomib therapy specifically for multiple myeloma. Its irreplaceable role in the disease's overall management makes multiple myeloma the dominant application segment within this market.
By End User- Ease of administration drives hospital sector as top end user
In terms of end user, hospitals segment is estimated to contribute the highest market share of, 52.5% in 2025 owing to their ideal setting for delivering chemotherapy treatments. While outpatient oncology clinics also commonly administer Bortezomib, hospitals offer important advantages for its use. Most myeloma patients require cycles of Bortezomib therapy in conjunction with other intravenously-delivered drug regimens. Hospitals are fully equipped with dedicated chemotherapy suites, clinical staff experienced in oncology infusion procedures, as well as inpatient facilities for managing side-effects or complications as needed.
Especially in disease settings where multiple treatment lines may be pursued such as relapsed/refractory multiple myeloma, hospitals provide seamless coordination of care through their specialized teams. These can monitor patients closely during Bortezomib treatment journeys that may span several months or longer. The larger practice scale of hospitals also translates to treatment access for patients across wider demographics through diverse locations. Many doctors prefer carrying out Bortezomib administration within hospitals' supportive environments. Reimbursement structures in several countries additionally favor hospital-based chemotherapy. These factors cumulatively make hospitals the prime end-use sector driving the majority of Bortezomib utilization worldwide.
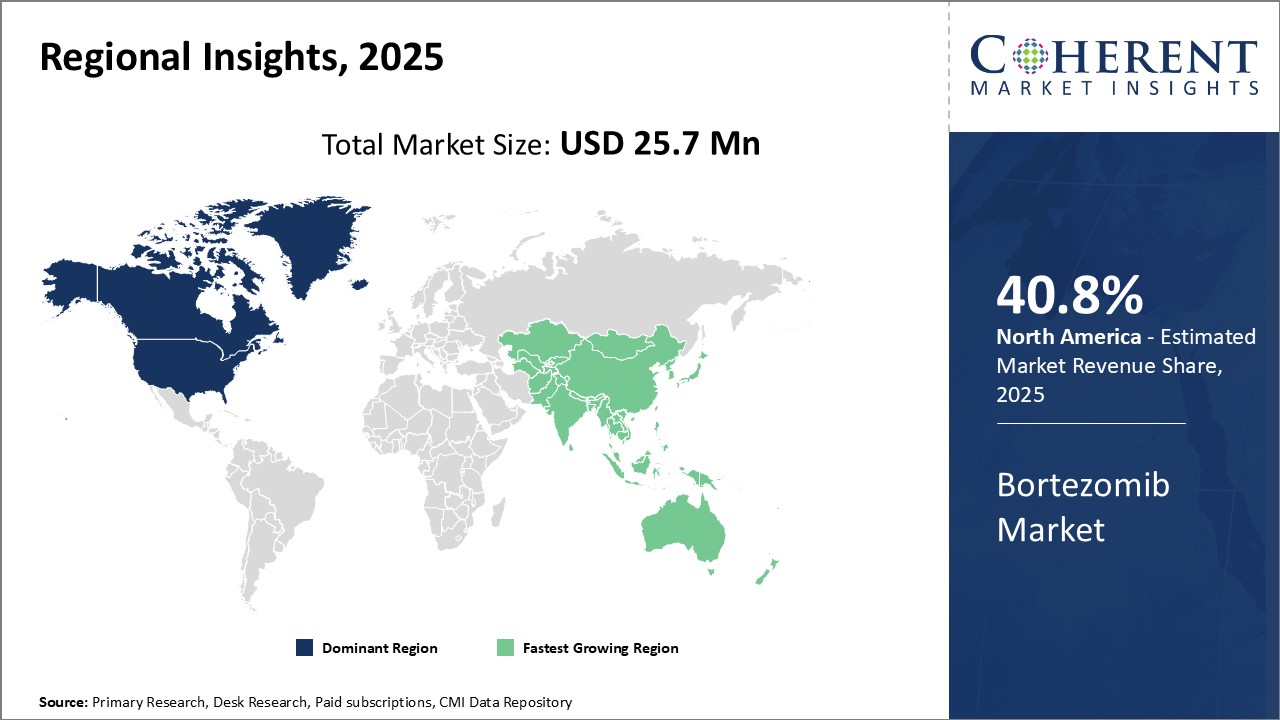
Regional Insights
Need a Different Region or Segment? Download Free Sample
North America dominates the global bortezomib market with share of 40.8% in 2025. The region is home to many of the largest biopharma companie,s which are actively engaged in the research and development of novel therapeutics for the treatment of various types of cancer including multiple myeloma. Due to strong presence of pharmaceutical industry in the U.S., there is ease of availability of latest treatment options for patients. Favorable regulatory environment and favorable reimbursement policies have ensured high adoption rate of innovative drugs in this region over generic equivalents.
Asia Pacific region, especially China and India, is considered as the fastest growing market for bortezomib. Steady economic growth over the past few decades in emerging nations of Asia has expanded the affordable healthcare access to large population. There is increasing healthcare expenditure by both public and private players. This has boosted demand for cost-effective cancer treatment options in the region. Growing geriatric population who are more prone to developing cancer can also drive the market growth. Local drug manufacturers have also started production of biosimilar bortezomib, providing cost competitive treatment alternative to innovator brands.
Emerging markets of Asia Pacific are importing substantial volume of bortezomib APIs and intermediates to cater to increasing demand from local pharmaceutical companies engaged in formulation development. Pricing of locally manufactured bortezomib products is much lower compared to developed markets making it affordable for greater number of patients. Governments are also taking initiatives to spread cancer treatment awareness and early detection programs to curb disease burden.
Market Report Scope
Bortezomib Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 25.7 Mn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 4.6% | 2032 Value Projection: | USD 35.2 Mn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Hikma Pharmaceuticals, Pfizer, Meitheal Pharmaceuticals, Novartis International AG, Bristol Myers Squibb, NATCO Pharma, Teva Pharmaceuticals, Dr. Reddy's Laboratories, Gland Pharma, Shilpa Medicare, Qilu Pharmaceutical, Scion Pharmaceuticals, Farmhispania Group, Coresyn, Chem-Stone (Guangzhou), Hubei Honch Pharmaceutical, Vinkem Labs, Icrom, TAPI Teva, Chengdu Aslee Biopharmaceuticals |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Bortezomib Industry News
- On July 30, 2024, the U.S. FDA approved daratumumab and hyaluronidase-fihj (DarzalexFaspro, Janssen Research & Development, LLC) in combination with bortezomib, lenalidomide, and dexamethasone for induction and consolidation therapy in newly diagnosed multiple myeloma patients eligible for autologous stem cell transplant (ASCT)
- In December 2023, Janssen-Cilag International NV announced new data at the 55th ASH Annual Meeting, highlighting VELCADE (bortezomib) as a key treatment for multiple myeloma. The data show that higher cumulative doses of VELCADE improve survival, significantly enhance response rates and slow disease progression when combined with dexamethasone, and are associated with low rates of cardiotoxicity.
- In November 2023, GSK plc announced positive results from the interim analysis of the DREAMM-7 phase III trial, which showed that belantamabmafodotin, combined with bortezomib and dexamethasone (BorDex), significantly extended progression-free survival (PFS) compared to daratumumab plus BorDex in patients with relapsed or refractory multiple myeloma. The trial also observed a strong trend toward improved overall survival (OS) with a nominal p-value < 0.0005, and follow-up for OS is ongoing.
*Definition: Global bortezomib market refer to therapeutic use of the proteasome inhibitor bortezomib, primarily for treating multiple myeloma and mantle cell lymphoma. This market encompasses the worldwide sales of bortezomib drugs, marketed under various brand names across different regions. Leading companies are engaged in the production and commercialization of bortezomib formulations, including injections and lyophilized powders for reconstitution.
Market Segmentation
- By Product Type Insights (Revenue, USD Mn, 2020 - 2032)
-
- Injection
- Powder for Injection
- By Application Insights (Revenue, USD Mn, 2020 - 2032)
- Multiple Myeloma
- Mantle Cell Lymphoma
- Other Hematological Malignancies
- By End User Insights (Revenue, USD Mn, 2020 - 2032)
- Hospitals
- Oncology Clinics
- Ambulatory Surgical Centers
- Others (Research Institutes, Cancer Centers)
- By Regional Insights (Revenue, USD Mn, 2020 - 2032)
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Key Players Insights
- Hikma Pharmaceuticals
- Pfizer
- Meitheal Pharmaceuticals
- Novartis International AG
- Bristol Myers Squibb
- NATCO Pharma
- Teva Pharmaceuticals
- Reddy's Laboratories
- Gland Pharma
- Shilpa Medicare
- Qilu Pharmaceutical
- Scion Pharmaceuticals
- Farmhispania Group
- Coresyn
- Chem-Stone (Guangzhou)
- Hubei Honch Pharmaceutical
- Vinkem Labs
- Icrom
- TAPI Teva
- Chengdu Aslee Biopharmaceuticals
Share
Share
About Author
Ghanshyam Shrivastava - With over 20 years of experience in the management consulting and research, Ghanshyam Shrivastava serves as a Principal Consultant, bringing extensive expertise in biologics and biosimilars. His primary expertise lies in areas such as market entry and expansion strategy, competitive intelligence, and strategic transformation across diversified portfolio of various drugs used for different therapeutic category and APIs. He excels at identifying key challenges faced by clients and providing robust solutions to enhance their strategic decision-making capabilities. His comprehensive understanding of the market ensures valuable contributions to research reports and business decisions.
Ghanshyam is a sought-after speaker at industry conferences and contributes to various publications on pharma industry.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients
