Global Bioburden Testing Market Size and Share Analysis – Growth Trends and Forecasts (2025-2032)
The bioburden testing market size is valued at USD 1.4 Bn in 2025 and is expected to reach USD 2.63 Bn by 2032, growing at a compound annual growth rate (CAGR) of 9.4% from 2025 to 2032.
Key Takeaways
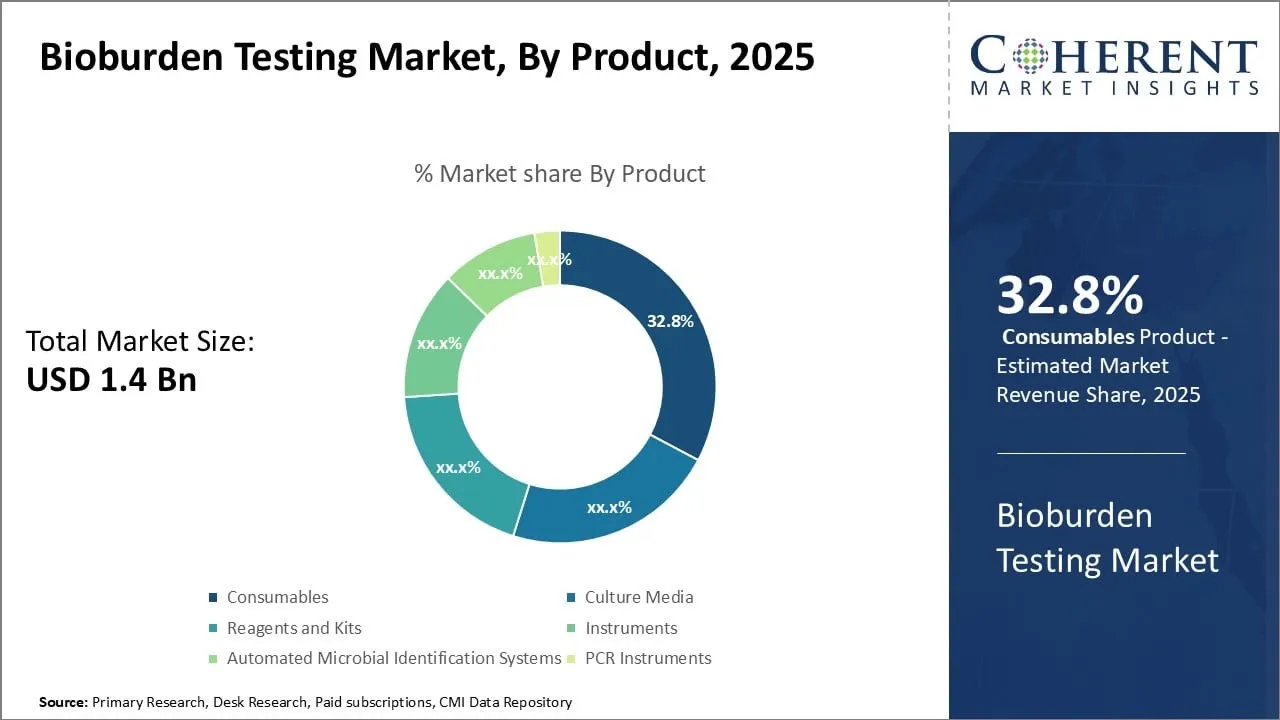
- Based on Product, Consumables Product Segment dominates the market with 32.8% market share. This is due to their recurring usage in every test, ensuring constant demand.
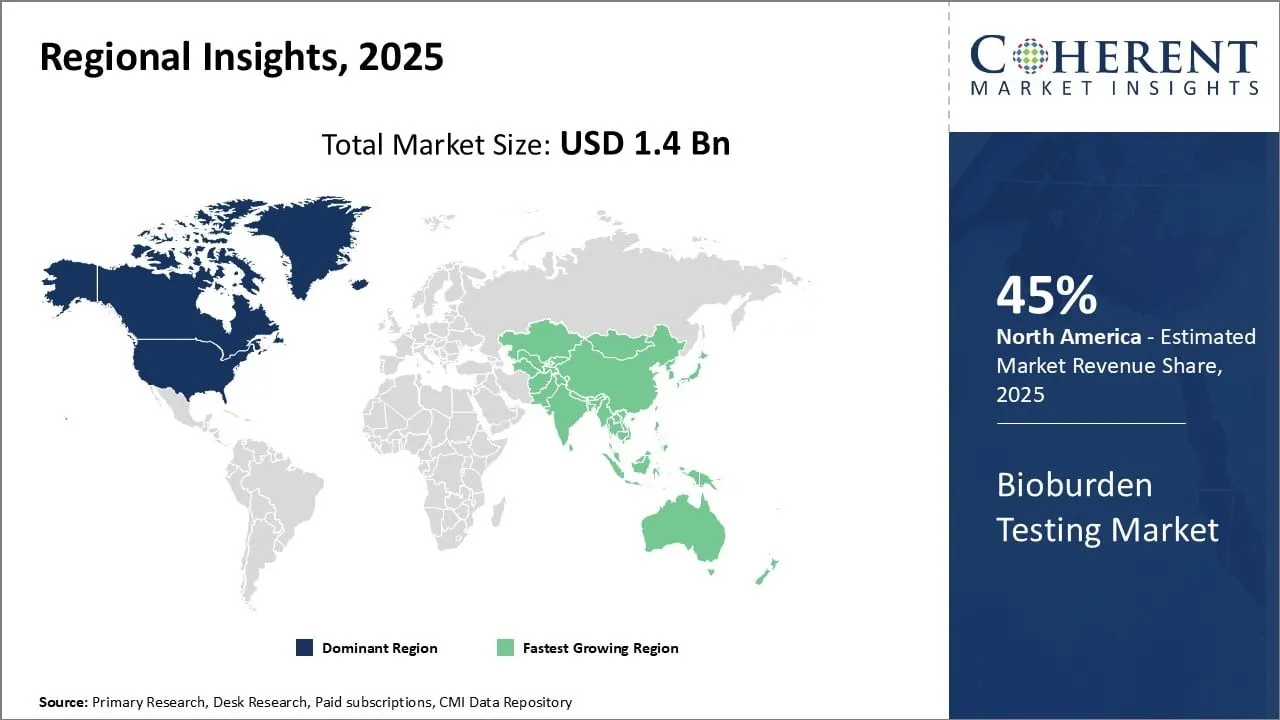
- North America is dominating the global bioburden testing market with a 45%market share and is expected to continue its leading position in the coming years. This due to the strong presence of pharmaceutical and biotech companies in countries like U.S.
Market Overview
The bioburden testing market is witnessing robust growth, driven by rising regulatory compliance requirements across the pharmaceutical, biotech, and medical device sectors. Growing sterility assurance requirement, especially in pharmaceutical manufacture and device manufacture, is a key driver of growth. Outsourcing microbial testing to CROs and CMOs is a trend that is growing rapidly, providing vendors with the ability to expand service-based product offerings. Implementation of RMMs, automation, and electronic data management is also changing traditional bioburden testing protocols, making them faster and more efficient.
In June 2024 BioMérieux, a world leader in in vitro diagnostic solutions, unveil its new-generation rapid bioburden testing platform—BIOFIRE® BC System—the first to leverage multiplex PCR and AI-based analytics to provide microbial detection results in less than a very short time of one hour, greatly facilitating pharma quality control decision-making.
Current Events and Its Impact on Bioburden Testing Market
|
Current Events |
Description and its impact |
|
FDA Widens Expectations for Fast Microbial Analysis in Pharma Manufacturing |
|
|
Charles River Increases Microbial Solutions Services in Singapore |
|
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Pricing Analysis
The global market for bioburden testing is increasing steadily, and its value is forecasted to be on the order of some USD 2.3–2.9 billion in 2030 from approximately USD 1.2–1.6 billion in 2024. The cost in this market is fixed by a number of factors, essentially divided between capital equipment, consumables, and service-based testing. Equipment used in bioburden testing—e.g., automated colony counters, filtration, and PCR-based systems—is typically high-priced investments, ranging from $100,000 to over $500,000 depending on automation and capacity.
These are typically barriers to entry for smaller businesses or facilities in the developing world, resulting in leasing and outsourcing modes. Reagents and consumables like growth-supporting additives, filters, and culture media are recurrent costs and typically cost between $5 and $20 per test. They are a significant component of recurrent running costs and play a critical role in revenue for long-term financial sustainability in suppliers.
There is also a pressure of pricing due to supply chain limitations worldwide, which sometimes have led to increased reagent prices. Most drug and medical device firms are now turning to third-party contract testing laboratories to avoid having to make up-front investments of tens of thousands of dollars, shifting the emphasis from capital to operating expense.
The contract labs bundle the cost of service and consumables together, typically at 20–40% markups. While instrument costs are expected to rise moderately because of integration with leading-edge features like AI and remote monitoring, cost reduction per test is a thrust area with bulk purchasing, automation, and high-throughput technologies helping reduce effective cost. Overall, the business model of the market is that of high initial investment against recurring, scalable consumable expense and outsourced services paradigms.
Role of Emerging Technologies
Emerging technologies in the bioburden testing market are transforming traditional microbial detection methods with a boost in speed, accuracy, and automation. The most relevant innovation is the combination of rapid microbiological techniques (RMMs), such as real-time PCR, flow cytometry, and ATP bioluminescence, which immensely shorten the time required for microbial enumeration from days to hours or minutes. Such technologies are being increasingly applied in pharmaceutical, biotechnology, and medical device firms due to their ability to meet stringent regulatory requirements while improving the operation efficiency.
Another major innovation is the use of automated microbial detection platforms, which combine robotics, smart sensors, and artificial intelligence-powered analytics to eliminate human error and increase throughput. Devices like automated colony counters and filtration-based platforms with onboard imaging are used to automate workflow in in-house labs as well as contract testing companies.
Lab-on-a-chip and microfluidic technologies also come into view as useful instruments for point-of-use, portable bioburden testing. These platforms offer microscaled, real-time detection with zero reagent consumption, which is especially well-suited for decentralized manufacturing facilities or field analysis.
Also, IoT integration and cloud-connected devices are enabling remote monitoring, compliance tracking, and data storage, which are extremely critical in regulated environments. These solutions not only simplify audits and traceability but also predictive maintenance of lab equipment.
Bioburden Testing Market Drivers
- Strict Regulations for Bioburden Testing
Government regulations play a huge role in driving the need for bioburden testing of pharmaceutical and medical device products. Regulatory bodies across the world have implemented stringent norms and guidelines to ensure product safety and quality.
For instance, the U.S. FDA has laid down clear specifications regarding microbial limits for parenteral and other medical products. Similarly, regulations in the EU and other developed markets also mandate testing for microbial contamination in manufacturing environments, raw materials, and finished medical goods.
In late 2024, Merck Life Science introduced a fully automated bioburden testing prototype integrating Milliflex Oasis® pumps capable of processing 240 filtrations in 12 hours. This system automates liquid handling, membrane integrity checks, incubation, and colony counting—boosting throughput, data traceability, and compliance in GMP environments.
Ensuring compliance with such regulatory standards has become imperative for companies. Even a single report of infection due to contamination can damage a brand’s reputation and incur heavy costs. Thus, manufacturers spend considerable time and resources on environmental monitoring in cleanrooms.
Sophisticated bioburden testing is done at various stages, ranging from raw material receipt to sterilization validation. Advanced automated equipment and rapid methods are employed to detect even the minutest microbial levels within set timelines. Any non-compliance can attract severe penalties and affect future clearances. This has made it important for quality control teams to rely on reliable and validated bioburden testing services to meet global quality norms.
- Growing Demand for Outsourcing of Testing Services
Bioburden testing requires specialized microbiological expertise and advanced laboratory infrastructure. However, setting up an in-house microbiology laboratory and maintaining it at global standards requires significant capital and recurring investments. It also involves constantly training staff on evolving testing techniques and compliance practices. For many mid-sized and smaller firms, establishing such facilities may not seem financially viable considering their production volumes.
At the same time, outsourcing testing needs are fraught with quality and timeline risks. There is a constant need to monitor service providers’ capabilities and ensure smooth coordination. However, in recent times, this challenge has been addressed by a growing number of Good Laboratory Practice (GLP) certified full-service contract research organizations (CROs) offering bioburden testing.
They provide a centralized lab setup and a team of well-qualified scientists for all types of microbiological testing needs. CROs help reduce set-up costs and eliminate the risks of unpredictable internal delays or non-compliances by regulated lab staff.
Bioburden Testing Market Opportunities
- Expanding pharma and biotech industries in emerging markets
The rapidly growing pharmaceutical and biotechnology industries in emerging markets like India, China, Brazil, and others present a major opportunity for expansion in bioburden testing services. These developing economies are witnessing substantial economic growth, which is enabling greater investments in healthcare and drug development.
For instance, as per the World Bank, total healthcare spending as a percentage of GDP has increased significantly in the last decade in many developing nations. Healthcare spending in India has risen from just 3.5% of GDP in 2000 to about 5% in 2020.
- Growing demand for non-pharma applications of bioburden testing
The increasing demand for bioburden testing in non-pharmaceutical applications represents a significant opportunity for growth in this industry. Bioburden testing is no longer limited to just pharmaceutical and medical device manufacturing, as industries such as food and beverage, cosmetics, aerospace, and automotive are now recognizing the importance of ensuring stringent quality control and safety. With a growing global population and rising incomes, the demand for processed and packaged foods and beverages is increasing rapidly.
Consumers are more aware of product quality and hygiene, placing greater pressure on brands to implement robust safety practices. Any contamination could lead to disease outbreaks and the loss of lives. The UN Food and Agriculture Organization (FAO) estimates that nearly 1 in 10 people fall ill every year from eating contaminated food.
Bioburden Testing Market Insights, By product
Based on product type, Consumables product dominates the market with 32.8% market share. This is due to their recurring usage in every test, ensuring constant demand. These include culture media, reagents, filters, and plates, which are essential for microbial growth and detection.
Unlike instruments, consumables have a shorter lifecycle and cannot be reused, leading to higher turnover. They are critical across various industries including pharmaceuticals, medical devices, and food safety.
Regional Insights
To learn more about this report, Download Free Sample
Bioburden Testing Market Regional Insights
- North America
North America has dominated the global bioburden testing market with a 45% presence and is expected to continue its leading position in the coming years. This can be attributed to the strong presence of pharmaceutical and biotech companies in countries like U.S The region is home to top companies in the pharmaceutical industry that are continuously focusing on new drug development and maintaining stringent quality standards.
This has propelled the demand for bioburden testing to ensure the safety and efficacy of drugs. In addition, established regulations regarding quality control and standardization by regulatory bodies like the FDA have mandated bioburden testing for various medical products, further supporting market growth.
- Europe
Europe holds the second position in terms of market share due to the presence of major drug makers. Stringent medical regulations set by the European Medical Agency are also driving the implementation of bioburden testing at various manufacturing stages.
- Asia Pacific
Asia Pacific is recognized as the fastest-growing regional market due to the expansion of the pharmaceutical industry in countries like China, India, and South Korea. Growing generic drug production as well as the presence of contract manufacturing organizations in these countries offer vast opportunities for bioburden testing providers.
Asia Pacific countries also emphasize on quality standards to ensure access to global markets. This has further catalyzed the adoption of bioburden testing solutions. Rising healthcare expenditure and increasing healthcare awareness have also played a crucial role in market development.
Many international players are establishing manufacturing facilities and collaborating with regional companies to leverage growth opportunities. This helps in tech transfer and developing testing infrastructure to cater to rising demand.
Bioburden Testing Market Dominating Countries
United States Bioburden Testing Market Analysis and Trend
The U.S. leads the world market for bioburden testing, driven by a strong pharma and biotech market, stringent FDA regulations, and extensive use of advanced test technologies. High R&D spends and presence of big market players drive it to become a hub of innovation and large-scale production.
Germany Bioburden Testing Market Analysis and Trend
Germany dominates the European market by having one of the most advanced medical device and drug-producing industries in the world. The country is committed to upholding stringent levels of quality and adherence to EU standards, which drives demand for consistent bioburden testing solutions across the industrial and clinical spaces.
China Bioburden Testing Market Analysis and Trend
China is also entering the bioburden testing market very quickly, propelled by its emerging pharmaceutical manufacturing base and increasing investment in healthcare infrastructure. Regulatory reforms and increased exports of medical devices are compelling adoption of next-generation microbiological testing technology.
India Bioburden Testing Market Analysis and Trend
India is also coming as a big market due to the growing rise of contract manufacturing organizations (CMOs) and a strong generics industry. The government initiative towards better regulatory requirements and higher pharmaceutical exports are all leading to a higher demand for high-throughput and standardized bioburden testing protocols.
Market Report Scope
Bioburden Testing Market Report Coverage
| Report Coverage | Details | ||
|---|---|---|---|
| Base Year: | 2024 | Market Size in 2025: | USD 1.4 Bn |
| Historical Data for: | 2020 To 2024 | Forecast Period: | 2025 To 2032 |
| Forecast Period 2025 to 2032 CAGR: | 9.4% | 2032 Value Projection: | USD 2.63 Bn |
| Geographies covered: |
|
||
| Segments covered: |
|
||
| Companies covered: |
Charles River Laboratories International, Inc., SGS SA, Merck KGaA, Becton Dickinson and Company, Wuxi Apptec, North American Science Associates Inc., Nelson Laboratories, LLC, Biomérieux SA, Thermo Fisher Scientific and Pacific Biolabs |
||
| Growth Drivers: |
|
||
| Restraints & Challenges: |
|
||
Uncover macros and micros vetted on 75+ parameters: Get instant access to report
Recent Developments
- In May 2024, Thermo Fisher Scientific launched the Sartorius Digital Bioburden Testing Platform, an AI-augmented system offering centralized sample management and automated data analytics. This platform integrates real-time environmental monitoring with intelligent colony counting, reducing manual workload and enhancing regulatory compliance, especially in high-throughput GMP settings.
- In March 2024, Charles River Laboratories unveiled a rapid sterility testing platform for pharmaceutical products that reduces test time from 14 days to just 5 days. This development addresses the pressing demand for faster, validated microbial testing aligned with stringent regulatory expectations.
Analyst Viewpoint
- The bioburden testing market is poised to grow substantially in the coming years. Rising regulatory standards for biopharmaceutical and medical device manufacturers to assess microbial contamination levels in raw materials and finished products have increased demand for bioburden testing.
- Additionally, the rapid expansion of the biopharmaceutical industry and growing generics market will drive increased compliance testing requirements, including the sterilization validation process, which is essential to ensure microbial safety in drug manufacturing. North America currently dominates the market due to well-established regulatory frameworks and a sizable biomanufacturing industry in the region. Asia Pacific is expected to be the fastest-growing market owing to positive economic and regulatory conditions accelerating biosimilars production in countries like China, India, and South Korea.
- However, high capital investment requirements for stringent pathogen testing instruments like PCR machines and automated rapid detection systems may restrain market growth potential to some extent, particularly among small and medium enterprises in price-sensitive developing markets. Increasing adoption of continuous monitoring systems instead of batch-wise testing could present an opportunity for suppliers to gain recurring revenue streams.
- The development of rapid, on-site bioburden testing technologies may appeal to producers seeking faster results without sacrificing accuracy, especially in the context of stringent product safety testing Outsourcing of testing services is anticipated to rise, as it relieves manufacturers of upfront resource and equipment costs while ensuring compliance with regulatory standards.
Market Segmentation
- Bioburden Testing Market, By Product
- Consumables
- Culture Media
- Reagents and Kits
- Instruments
- Automated Microbial Identification Systems
- PCR Instruments
- Bioburden Testing Market, By Test Type
- Aerobic Count Testing
- Anaerobic Count Testing
- Fungi/Mold Count Testing
- Spore Count Testing
- Bioburden Testing Market, By Application
- Raw Material Testing
- Medical Device Testing
- In-Process Material Testing
- Sterilization Validation Testing
- Equipment Cleaning Validation
- Bioburden Testing Market, By End Use
- Pharmaceutical & Biotechnology Companies
- Medical Device Manufacturers
- Contract Manufacturing Organizations
- Manufacturers of Food & Beverage and Agricultural Products
- Microbial Testing Laboratories
- Bioburden Testing Market, By Region
- North America
- U.S.
- Canada
- Latin America
- Brazil
- Argentina
- Mexico
- Rest of Latin America
- Europe
- Germany
- U.K.
- Spain
- France
- Italy
- Russia
- Rest of Europe
- Asia Pacific
- China
- India
- Japan
- Australia
- South Korea
- ASEAN
- Rest of Asia Pacific
- Middle East
- GCC Countries
- Israel
- Rest of Middle East
- Africa
- South Africa
- North Africa
- Central Africa
- North America
- Top Companies in the Bioburden Testing Market
- Charles River Laboratories International Inc.
- SGS SA
- Merck KGaA
- Becton
- Dickinson and Company
- Wuxi Apptec
- North American Science Associates Inc.
- Nelson Laboratories LLC
- Biomérieux SA,
- Thermo Fisher Scientific
- Pacific Biolabs.
Sources
Primary Research Interviews
- Medical Device Manufacturers
- Pharmaceutical Companies
- Contract Testing Laboratories
- Healthcare Regulatory Affairs Professionals
- Others
Databases
- FDA Database (U.S. Food and Drug Administration)
- EMA Database (European Medicines Agency)
- WHO Global Health Observatory
- Others
Magazines
- Pharmaceutical Technology Magazine
- BioPharm International
- Medical Device & Diagnostic Industry (MD+DI)
- Laboratory Equipment Magazine
- Cleanroom Technology Magazine
- Others
Journals
- Journal of Pharmaceutical Sciences
- Applied and Environmental Microbiology
- International Journal of Pharmaceutics
- Others
Newspapers
- Financial Times
- The Wall Street Journal
- Reuters Health News
- BioPharma Dive
- FierceBiotech
- Others
Associations
- International Organization for Standardization (ISO)
- Association for the Advancement of Medical Instrumentation (AAMI)
- Pharmaceutical Research and Manufacturers of America (PhRMA)
- European Federation of Pharmaceutical Industries and Associations (EFPIA)
- American Society for Microbiology (ASM)
- Others
Public Domain Sources
- U.S. Centers for Disease Control and Prevention (CDC)
- World Health Organization (WHO)
- National Institute of Standards and Technology (NIST)
- European Centre for Disease Prevention and Control (ECDC)
- Health Canada
- Others
Proprietary Elements
- CMI Data Analytics Tool
- Proprietary CMI Existing Repository of information for last 8 years
*Definition: Bioburden testing is a crucial quality control process that measures the levels of microbial contamination in various products, including water, raw materials, and finished products. It is performed for a wide range of items, such as medical devices, pharmaceuticals, food and beverages, water, packaging, raw materials, human tissue, animal tissue, and cosmetics. The aim of bioburden testing is to ensure the safety, quality, and regulatory compliance of each manufactured product batch. It involves detecting and quantifying the presence of viable microorganisms before sterilization, as these can be introduced from raw materials or through the manufacturing process. The testing methods include direct plating methods such as the pour plate and spread plate methods, with the pour plate method being preferred due to its higher theoretical accuracy.
Share
Share
About Author
Abhijeet Kale is a results-driven management consultant with five years of specialized experience in the biotech and clinical diagnostics sectors. With a strong background in scientific research and business strategy, Abhijeet helps organizations identify potential revenue pockets, and in turn helping clients with market entry strategies. He assists clients in developing robust strategies for navigating FDA and EMA requirements.
Missing comfort of reading report in your local language? Find your preferred language :
Transform your Strategy with Exclusive Trending Reports :
Frequently Asked Questions
EXISTING CLIENTELE
Joining thousands of companies around the world committed to making the Excellent Business Solutions.
View All Our Clients